The Ultimate Guide: P Valence Electrons
Understanding the concept of valence electrons is crucial in the realm of chemistry and atomic structure. In this comprehensive guide, we will delve into the intricacies of p valence electrons, exploring their role, characteristics, and significance in chemical bonding and reactivity. By the end of this article, you will have a profound understanding of p valence electrons and their impact on the periodic table and chemical compounds.
Unveiling the World of Valence Electrons
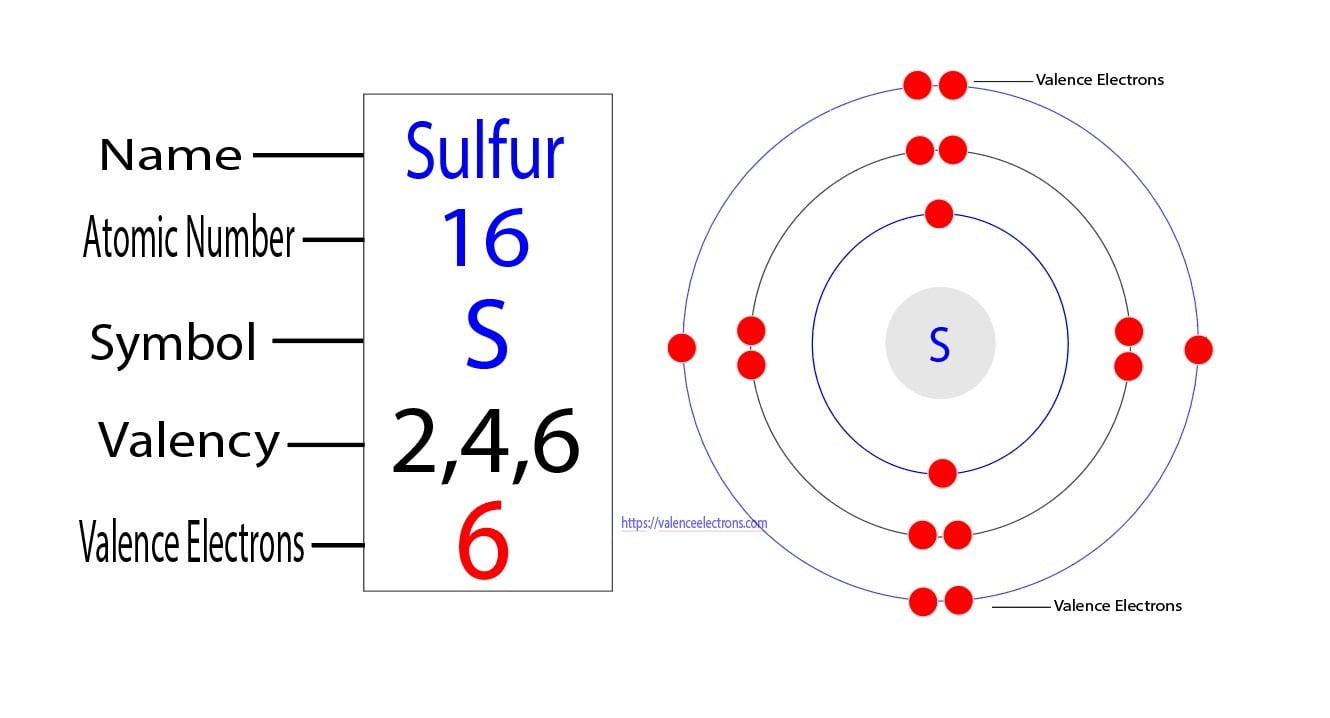
Valence electrons, often referred to as the outermost electrons of an atom, play a pivotal role in determining an atom’s chemical behavior. These electrons are located in the atom’s outermost energy level or shell, also known as the valence shell. The number and arrangement of valence electrons significantly influence an atom’s ability to bond with other atoms and its overall reactivity.
In the periodic table, atoms are organized based on their atomic number, which corresponds to the number of protons in their nucleus. This arrangement reflects the increasing energy levels and the filling of electron shells as we move across the table from left to right and down the columns.
When examining the electronic configuration of atoms, we find that the p-block elements, occupying the rightmost portion of the periodic table, exhibit a unique pattern in their valence electrons. These elements, including non-metals like oxygen, nitrogen, and carbon, possess electrons in the p orbital, hence the term p valence electrons. Understanding the properties and behavior of p valence electrons is essential for comprehending the reactivity and bonding tendencies of these elements.
The Nature of p Valence Electrons
p Valence electrons are located in the p orbital, which is one of the four types of orbitals (s, p, d, and f) present in an atom’s outermost energy level. The p orbital is a region of space around the nucleus where the probability of finding electrons is highest. It is characterized by its shape, which resembles a dumbbell or a pair of lobed regions.
Each p orbital can accommodate a maximum of six electrons. These electrons are typically found in pairs, as they prefer to occupy the lowest energy levels available. When an atom has a full set of six p electrons, it becomes more stable and less reactive, a concept known as the octet rule. However, the tendency to attain a stable electronic configuration varies among different p-block elements.
| Element | p Valence Electrons |
|---|---|
| Boron (B) | 3 |
| Carbon (C) | 4 |
| Nitrogen (N) | 5 |
| Oxygen (O) | 6 |
| Fluorine (F) | 7 |
For instance, consider the case of carbon, which has four valence electrons in its p orbital. Carbon's unique ability to form strong covalent bonds with itself and other elements makes it a fundamental building block in organic chemistry. Its tetravalent nature allows it to participate in a wide range of chemical reactions, leading to the formation of diverse organic compounds.
Bonding and Reactivity of p Valence Electrons
The presence of p valence electrons significantly influences the bonding and reactivity of p-block elements. These electrons are highly involved in the formation of chemical bonds, primarily through covalent bonding. Covalent bonds occur when two atoms share electrons, often to achieve a stable electronic configuration resembling that of a noble gas.
For example, in the case of nitrogen (N), with five valence electrons, it tends to form multiple bonds with other atoms to achieve a stable octet. Nitrogen molecules (N2) consist of a triple bond between two nitrogen atoms, sharing three pairs of electrons. This strong bond contributes to nitrogen's stability and its role as a crucial component in biological systems.
Furthermore, the reactivity of p-block elements is closely tied to the availability and distribution of their p valence electrons. Elements with fewer p electrons, such as boron (B) with three valence electrons, are often highly reactive and tend to form strong bonds with other elements. In contrast, elements with a full set of six p electrons, like oxygen (O), are less reactive and prefer to form multiple bonds with other atoms to satisfy their octet rule.
Exploring the Periodic Table: Trends in p Valence Electrons
The periodic table provides a systematic arrangement of elements based on their atomic number and electronic configuration. When examining the p-block elements, several trends emerge regarding their p valence electrons.
Group Trends
Elements within the same group of the periodic table share similar chemical properties due to their identical valence electron configurations. For instance, group 17, known as the halogens, consists of elements such as fluorine (F), chlorine (Cl), and bromine (Br). All these elements have seven valence electrons in their p orbital, making them highly reactive and prone to gaining an electron to achieve a stable octet.
Period Trends
As we move across a period from left to right, the number of p valence electrons increases by one for each element. This trend reflects the filling of the p orbital with electrons as we progress from the first period to the sixth period in the periodic table. For example, in period 2, we find elements like lithium (Li) with one valence electron, while in period 3, we encounter elements like phosphorus (P) with five valence electrons.
The Role of p Valence Electrons in Chemical Compounds
p Valence electrons are instrumental in the formation and stability of various chemical compounds. The ability of atoms to share, gain, or lose electrons through bonding determines the chemical nature and properties of these compounds.
Covalent Bonding
Covalent bonding is a common type of chemical bond involving the sharing of p valence electrons. Atoms with similar electronegativity values tend to form covalent bonds, where electrons are shared equally between the atoms. This sharing leads to the formation of stable molecules with unique properties.
For instance, consider the carbon dioxide (CO2) molecule. Carbon, with its four valence electrons, forms double covalent bonds with two oxygen atoms, each possessing six valence electrons. The equal sharing of electrons results in a stable and non-polar molecule.
Ionic Bonding
In some cases, p valence electrons can be transferred from one atom to another, leading to the formation of ionic bonds. This type of bonding occurs between atoms with significantly different electronegativity values. The atom with a higher electronegativity tends to attract the electrons of the other atom, resulting in the formation of ions.
An example of this is the sodium chloride (NaCl) molecule. Sodium (Na), with one valence electron, readily donates this electron to chlorine (Cl), which has seven valence electrons. This transfer results in the formation of a sodium ion (Na+) and a chloride ion (Cl−), held together by electrostatic attraction.
Future Implications and Applications
The understanding of p valence electrons and their behavior has profound implications for various scientific and technological advancements.
In the field of materials science, the manipulation of p valence electrons can lead to the development of novel materials with unique electronic and optical properties. For instance, the study of transition metal oxides, where p valence electrons play a crucial role, has resulted in the creation of materials with applications in electronics, energy storage, and catalysis.
Additionally, the concept of p valence electrons is essential in the design and synthesis of organic compounds. Chemists utilize the knowledge of electron distribution and reactivity to create complex molecules with specific biological or medicinal activities. This has led to the development of innovative pharmaceuticals and targeted therapies.
Conclusion
In this comprehensive guide, we have unraveled the mysteries of p valence electrons, their nature, and their impact on chemical bonding and reactivity. From their role in the periodic table to their involvement in the formation of diverse chemical compounds, p valence electrons are a fundamental concept in the realm of chemistry.
Understanding the intricacies of p valence electrons allows scientists and researchers to manipulate and harness their potential, leading to groundbreaking discoveries and technological advancements. As we continue to explore the world of chemistry, the knowledge of p valence electrons will undoubtedly play a pivotal role in shaping our understanding of the atomic and molecular world.
How many p valence electrons does a carbon atom have?
+A carbon atom has four valence electrons in its p orbital. This tetravalent nature allows carbon to form strong covalent bonds with itself and other elements, leading to the formation of diverse organic compounds.
What is the octet rule, and how does it relate to p valence electrons?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electronic configuration resembling that of a noble gas, which has a full set of eight valence electrons. p Valence electrons play a crucial role in achieving this stability, as elements strive to fill their p orbitals to attain an octet.
Can p valence electrons be involved in both covalent and ionic bonding?
+Yes, p valence electrons can participate in both covalent and ionic bonding. In covalent bonding, p valence electrons are shared equally between atoms, forming strong covalent bonds. In ionic bonding, p valence electrons are transferred from one atom to another, resulting in the formation of ions.


