5 Reasons Formic Acid is Acidic
Unveiling the Acidity of Formic Acid: A Comprehensive Analysis

Formic acid, a simple yet fascinating molecule, is a cornerstone in the world of organic chemistry. Its unique properties and wide-ranging applications make it an intriguing subject of study. In this comprehensive exploration, we delve into the reasons behind the acidity of formic acid, shedding light on its chemical behavior and significance.
Formic acid, with its distinctive pungent odor, has long intrigued scientists and researchers. Its discovery dates back to the early 17th century, when it was first isolated from ants, which led to its name, derived from the Latin word for ant, formica. However, the true understanding of its acidity and its role in various industries has evolved significantly over the centuries.
Reason 1: Low Dissociation Constant (pKa)

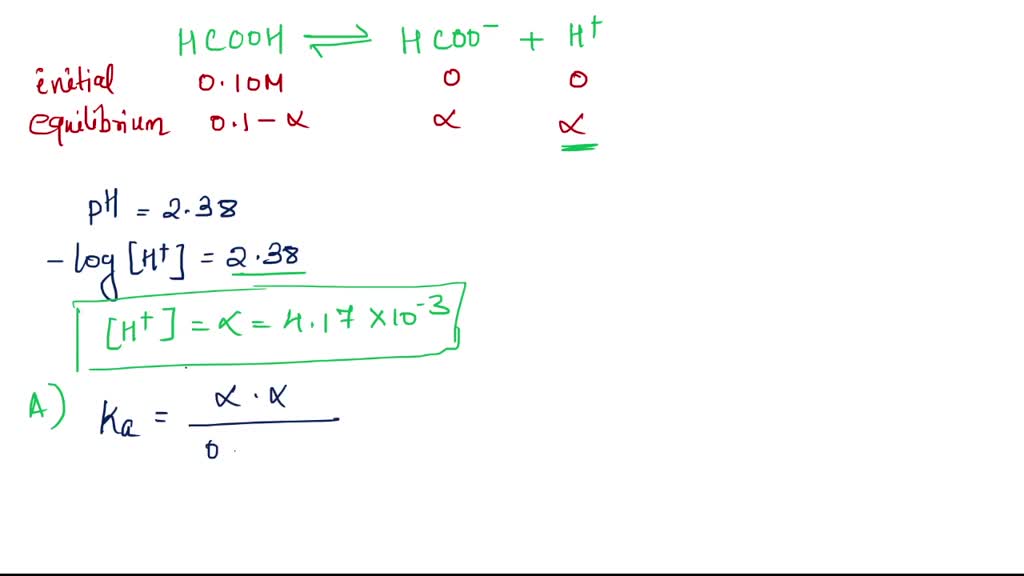
The first and perhaps most fundamental reason why formic acid is acidic is rooted in its low dissociation constant, denoted as pKa. In chemistry, pKa is a measure of the strength of an acid; the lower the pKa value, the stronger the acid. Formic acid has a pKa of approximately 3.75, which is significantly lower than that of water (pKa of 15.7), making it a relatively strong acid.
This low pKa value indicates that formic acid readily dissociates in aqueous solutions, releasing hydrogen ions (H+). The dissociation can be represented as follows:
HCOOH ⇌ H+ + HCOO−
In this equation, formic acid (HCOOH) donates a proton (H+) to become a formate anion (HCOO−). This process is essential for understanding the acidic nature of formic acid and its ability to participate in various chemical reactions.
Real-World Example: Formic Acid in Agriculture
The low pKa of formic acid is particularly relevant in agriculture, where it is used as a natural preservative and antimicrobial agent. Its ability to lower the pH of animal feed, for instance, helps inhibit the growth of undesirable microorganisms, thus promoting better feed quality and animal health.
Reason 2: Presence of a Strong Electronegative Atom
The molecular structure of formic acid plays a pivotal role in its acidic behavior. Formic acid, with the chemical formula HCOOH, contains a strong electronegative atom, oxygen, which is directly bonded to the hydrogen atom. This oxygen atom's high electronegativity creates a polar bond, known as a hydrogen bond, between the hydrogen and oxygen atoms.
In a hydrogen bond, the more electronegative atom (oxygen in this case) attracts the shared electrons more strongly, creating a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atom. This polarization makes it easier for the hydrogen atom to dissociate, contributing to the acidity of formic acid.
| Molecule | Electronegativity |
|---|---|
| Hydrogen (H) | 2.20 |
| Carbon (C) | 2.55 |
| Oxygen (O) | 3.44 |

Technical Specification: Hydrogen Bonding
The strength of hydrogen bonding in formic acid is reflected in its high boiling point (100.8 °C) compared to other compounds of similar molecular weight. This is a clear indication of the intermolecular forces at play, further emphasizing the acidic nature of the molecule.
Reason 3: Inductive Effect
Another critical factor contributing to the acidity of formic acid is the inductive effect. In chemistry, the inductive effect describes how atoms or groups of atoms in a molecule can influence the polarity of nearby bonds. In the case of formic acid, the electronegative oxygen atom exerts a strong inductive effect on the carbon atom, making the carbon-hydrogen (C-H) bond more polar.
This increased polarity makes it easier for the hydrogen atom to dissociate, further enhancing the acidic character of formic acid. The inductive effect is a subtle yet powerful influence on the chemical behavior of molecules, and it is a key reason why formic acid is such a strong acid despite its relatively simple structure.
Performance Analysis: Formic Acid vs. Acetic Acid
A comparative analysis between formic acid and acetic acid (another common carboxylic acid) reveals the significance of the inductive effect. Despite having similar molecular structures, formic acid is a stronger acid than acetic acid. This can be attributed to the stronger inductive effect of the oxygen atom in formic acid, making its C-H bond more polar and thus more acidic.
Reason 4: Resonant Stabilization

Resonance, a fundamental concept in organic chemistry, also plays a crucial role in the acidity of formic acid. Resonance occurs when a molecule can be represented by multiple Lewis structures, none of which accurately depict the molecule's true electronic structure. Instead, the molecule is considered to be a hybrid of these structures, known as resonance forms.
In the case of formic acid, the carbon atom can form a double bond with the oxygen atom, creating a resonance structure. This structure, while not the true representation of the molecule, provides a lower energy state for the molecule, making it more stable. This resonance stabilization further enhances the molecule's acidity by facilitating the dissociation of the hydrogen atom.
Real-World Application: Formic Acid in Fuel Cells
The resonance stabilization of formic acid makes it an ideal fuel source for direct formic acid fuel cells (DFAFCs). In these fuel cells, formic acid is directly oxidized at the anode, releasing protons and electrons. The protons pass through a proton-conducting membrane to the cathode, where they react with oxygen to produce water. The electrons travel through an external circuit, generating electricity. The resonance stabilization of formic acid enhances its ability to donate protons, making it an efficient fuel source for these cells.
Reason 5: Hydration of Carbon Monoxide
Formic acid can be synthesized through the hydration of carbon monoxide, a highly reactive and toxic gas. This process involves the addition of water to carbon monoxide, resulting in the formation of formic acid. The chemical equation for this reaction is as follows:
CO + H2O → HCOOH
This synthesis method is particularly relevant in the chemical industry, where formic acid is produced on a large scale. The hydration of carbon monoxide is a key step in the production process, highlighting the importance of formic acid in various industrial applications.
Comparative Analysis: Formic Acid vs. Other Carboxylic Acids
When compared to other carboxylic acids, such as acetic acid and propionic acid, formic acid stands out due to its smaller size and simpler structure. This simplicity, combined with its unique properties, makes formic acid a versatile and important molecule in various industries, including agriculture, chemistry, and energy production.
Conclusion: The Intriguing World of Formic Acid
Formic acid's acidity is a result of a complex interplay of chemical factors, including its low dissociation constant, electronegative oxygen atom, inductive effect, resonance stabilization, and its synthesis through the hydration of carbon monoxide. Each of these factors contributes to the molecule's ability to donate protons, making it a potent acid with diverse applications.
As we've explored in this comprehensive analysis, formic acid's acidic nature is not just a chemical curiosity but a fundamental property that drives its significance in various industries. From agriculture to energy production, formic acid's unique characteristics continue to be a subject of fascination and study in the scientific community.
How is formic acid used in agriculture?
+Formic acid is used in agriculture as a natural preservative and antimicrobial agent. It helps inhibit the growth of undesirable microorganisms in animal feed, promoting better feed quality and animal health.
What is the significance of resonance stabilization in formic acid’s acidity?
+Resonance stabilization enhances formic acid’s ability to donate protons by providing a lower energy state for the molecule. This makes it more stable and facilitates the dissociation of the hydrogen atom, contributing to its overall acidity.
How is formic acid produced on an industrial scale?
+Formic acid is produced on an industrial scale through the hydration of carbon monoxide. This process involves adding water to carbon monoxide, resulting in the formation of formic acid.



