5 Ways to Master Specific Heat Solving
Understanding specific heat and its applications is crucial in various scientific and engineering fields. Specific heat, a fundamental concept in thermodynamics, describes the amount of heat energy required to raise the temperature of a substance by a certain degree. In this comprehensive guide, we will delve into the world of specific heat and explore five effective strategies to master its solving techniques. By following these methods, you'll gain the skills needed to tackle specific heat problems with confidence and accuracy.
1. Grasp the Conceptual Foundation of Specific Heat

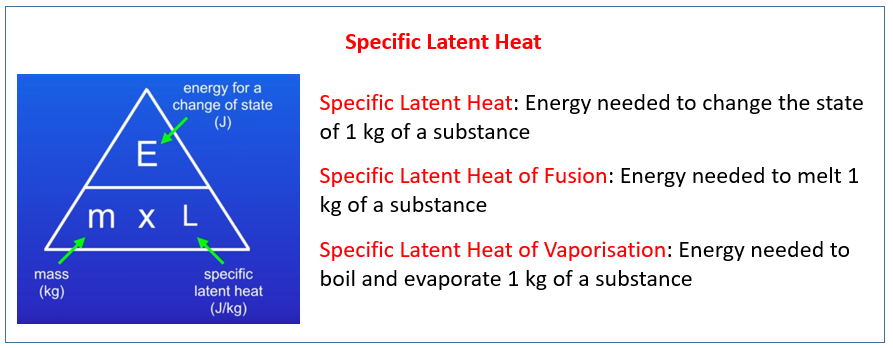
Before diving into the intricacies of specific heat solving, it’s essential to establish a solid conceptual understanding. Specific heat, often denoted as c, represents the heat capacity of a substance per unit mass. It quantifies how much heat energy is needed to increase the temperature of a given mass of the substance by one degree Celsius or Kelvin. This property is unique to each substance and plays a pivotal role in heat transfer processes.
To grasp the concept further, consider the following example. Water, with a specific heat capacity of approximately 4.18 J/g°C, requires more heat energy to raise its temperature compared to a substance like aluminum, which has a specific heat capacity of around 0.903 J/g°C. This means that water has a higher resistance to temperature changes, making it an excellent heat reservoir.
Understanding the relationship between specific heat, mass, temperature change, and heat energy is fundamental. The formula Q = m * c * ΔT encapsulates this relationship, where Q represents the heat energy transferred, m is the mass of the substance, c is the specific heat capacity, and ΔT denotes the change in temperature.
Subsection: The Role of Specific Heat in Various Applications
Specific heat finds extensive applications across multiple domains. In meteorology, it influences the behavior of atmospheric gases, impacting weather patterns. In engineering, specific heat is crucial for designing efficient heating and cooling systems. Additionally, specific heat plays a vital role in the field of thermodynamics, enabling the analysis of energy transfer in various processes.
| Application | Specific Heat Impact |
|---|---|
| Meteorology | Influences atmospheric behavior and weather conditions. |
| Engineering | Critical for designing efficient thermal systems. |
| Thermodynamics | Enables analysis of energy transfer in various processes. |

2. Master the Art of Dimensional Analysis

Dimensional analysis is a powerful tool that ensures the accuracy of calculations and problem-solving in physics and engineering. When tackling specific heat problems, mastering dimensional analysis is essential to avoid common errors and ensure the validity of your solutions.
Dimensional analysis involves breaking down the units of a physical quantity into their fundamental components. For specific heat, this means understanding the dimensions of mass, temperature, and heat energy. By analyzing these dimensions, you can set up consistent equations and perform unit conversions accurately.
Subsection: Dimensional Analysis in Action
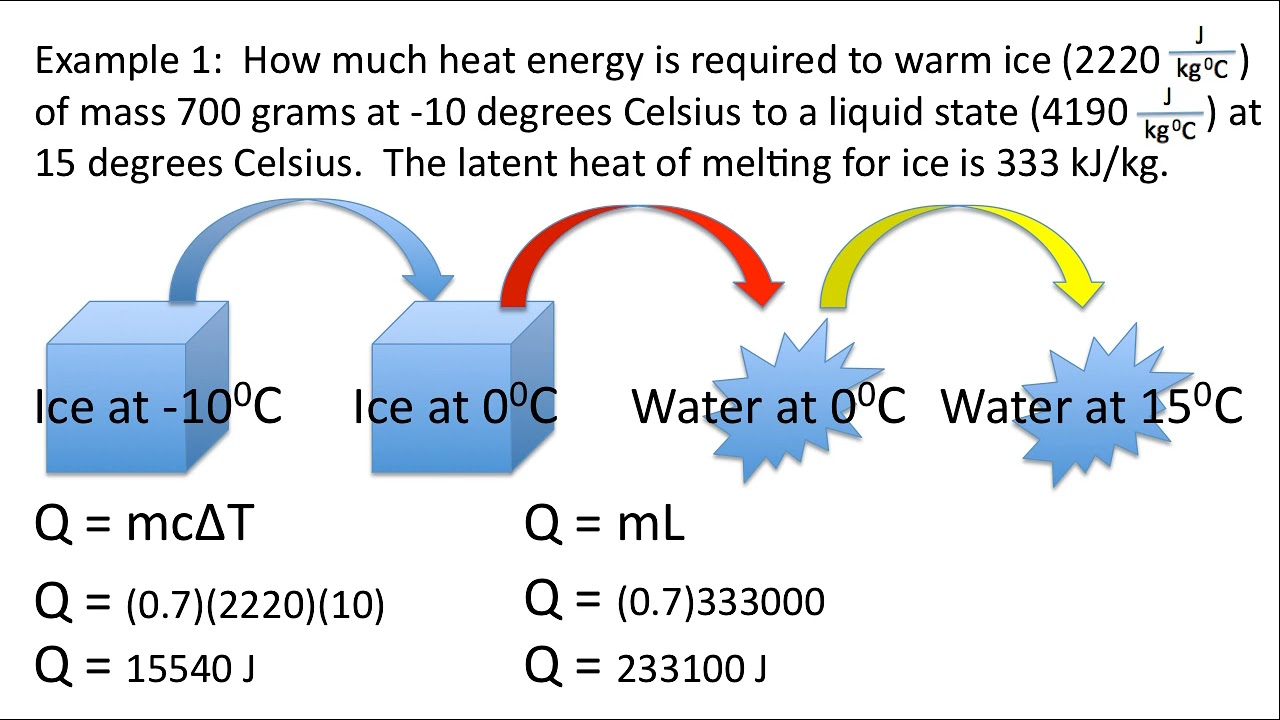
Let’s illustrate dimensional analysis with a practical example. Consider a specific heat problem where you need to calculate the heat energy required to raise the temperature of a 100-gram sample of water from 20°C to 80°C. The specific heat capacity of water is given as 4.18 J/g°C.
Using dimensional analysis, you can set up the equation as follows:
Q = m * c * ΔT
Where Q is the heat energy, m is the mass (100 grams), c is the specific heat capacity (4.18 J/g°C), and ΔT is the change in temperature (80°C - 20°C = 60°C). By plugging in the values and performing the calculations, you'll find the heat energy required for this process.
Dimensional analysis ensures that your calculations maintain consistency and that the units of your final answer are correct. It's a valuable skill to develop when working with specific heat and other physical quantities.
3. Explore Practical Examples and Real-World Scenarios
Specific heat is not just a theoretical concept; it has tangible applications in our everyday lives and various industries. By exploring practical examples and real-world scenarios, you can gain a deeper understanding of how specific heat affects different systems and processes.
Subsection: Specific Heat in Everyday Life
One common example is the use of specific heat in cooking. When heating a pot of water on the stove, the specific heat of water plays a crucial role in determining how long it takes to reach a boiling point. Similarly, the specific heat of different foods affects cooking times and the retention of heat during cooking.
Another practical application is in the field of engineering, where specific heat is essential for designing efficient heating and cooling systems. For instance, understanding the specific heat of different materials is vital when designing heat exchangers, ensuring optimal heat transfer and energy efficiency.
Subsection: Specific Heat in Industrial Processes
In industrial settings, specific heat is critical for various processes. For example, in the chemical industry, specific heat is used to control reaction temperatures and optimize energy usage. In the manufacturing of glass and ceramics, specific heat influences the behavior of materials during heating and cooling, impacting the final product’s quality.
Exploring these real-world applications of specific heat not only enhances your understanding of the concept but also showcases its practical significance across different industries.
4. Practice Problem-Solving with Varied Exercises
The key to mastering specific heat solving is through consistent practice. Engaging with a diverse range of exercises and problem sets will not only reinforce your understanding but also help you develop a systematic approach to solving complex problems.
Subsection: Building a Comprehensive Practice Routine
To create an effective practice routine, start with basic problems that involve calculating specific heat values or heat energy using the fundamental formula Q = m * c * ΔT. As you progress, introduce more challenging exercises that involve multiple substances, different units, and real-world scenarios.
For example, consider a problem where you need to determine the heat energy required to raise the temperature of a 500-gram aluminum block from 25°C to 75°C. With the specific heat capacity of aluminum being approximately 0.903 J/g°C, you can apply the formula and calculate the heat energy transferred.
Additionally, practice problems that involve finding specific heat values given heat energy and mass changes can further solidify your understanding. The more you practice, the more comfortable you'll become with solving specific heat problems, and the quicker you'll be able to apply the appropriate formulas and techniques.
5. Utilize Online Resources and Interactive Tools
In today’s digital age, numerous online resources and interactive tools are available to enhance your learning experience and make mastering specific heat more engaging and accessible.
Subsection: Online Tutorials and Video Lessons
Online platforms such as YouTube and educational websites offer a wealth of video tutorials and step-by-step guides on specific heat. These resources provide visual explanations, practical examples, and clear instructions to help you grasp the concepts and techniques more effectively.
For instance, you can find video tutorials that demonstrate how to calculate specific heat using real-world examples, breaking down the process into simple steps. These resources often include visual aids and animations to make the learning process more interactive and engaging.
Subsection: Interactive Simulations and Practice Platforms
Interactive simulations and practice platforms can further enhance your learning by providing a hands-on experience. These tools allow you to experiment with different scenarios, adjust variables, and observe the impact on specific heat calculations in real-time.
There are online platforms specifically designed for physics and engineering education that offer interactive simulations and problem sets focused on specific heat. These platforms often provide instant feedback, allowing you to identify and correct mistakes, thereby improving your problem-solving skills.
Subsection: Online Communities and Forums for Discussion
Engaging with online communities and forums dedicated to physics and engineering can be immensely beneficial. These platforms provide a space for discussing specific heat concepts, sharing insights, and seeking clarification on challenging problems. Interacting with fellow learners and experts can enhance your understanding and provide new perspectives.
Additionally, online forums often have a wealth of resources, including study guides, practice problems, and solved examples, which can serve as valuable references and learning aids.
What is specific heat, and why is it important to study?
+Specific heat, denoted as c, represents the heat capacity of a substance per unit mass. It quantifies the amount of heat energy required to raise the temperature of a substance by a certain degree. Studying specific heat is important because it provides insights into the behavior of substances under heat transfer, influencing various scientific and engineering applications.
How is specific heat related to heat energy and temperature change?
+Specific heat is directly related to heat energy and temperature change. The formula Q = m * c * ΔT encapsulates this relationship, where Q is the heat energy transferred, m is the mass of the substance, c is the specific heat capacity, and ΔT represents the change in temperature. This formula allows us to calculate heat energy or specific heat values based on temperature changes.
What are some common applications of specific heat in real-world scenarios?
+Specific heat has numerous real-world applications. In everyday life, it affects cooking times and the retention of heat in food. In engineering, it’s crucial for designing efficient heating and cooling systems. Industrial processes, such as chemical reactions and material manufacturing, also rely on specific heat to control temperatures and optimize energy usage.
How can dimensional analysis help with specific heat calculations?
+Dimensional analysis is a valuable tool for ensuring the accuracy of specific heat calculations. By breaking down units into their fundamental components, dimensional analysis helps set up consistent equations and perform unit conversions accurately. It ensures that your calculations maintain proper dimensions and units, leading to valid solutions.
Are there online resources and tools available to aid in learning specific heat solving techniques?
+Absolutely! Online platforms offer a wide range of resources, including video tutorials, interactive simulations, and practice platforms specifically designed for learning specific heat solving techniques. These resources provide visual explanations, hands-on practice, and instant feedback, making the learning process more engaging and effective.



