5 Ways: HCl and NaOH Reactions
In the world of chemistry, the interaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a fundamental and fascinating reaction. This simple yet crucial process has wide-ranging implications in various industries and scientific disciplines. The formation of salt and water through the combination of these two substances is not just a chemical curiosity; it's a cornerstone of chemical understanding and practical application.
This article will delve into the intricacies of the HCl-NaOH reaction, exploring its characteristics, mechanisms, and real-world applications. By the end, you'll have a comprehensive understanding of this reaction and its significance.
The Nature of HCl and NaOH

Hydrochloric acid, a strong mineral acid, is a colorless, highly corrosive solution of hydrogen chloride (HCl) gas in water. It is a versatile and essential compound, widely used in the chemical industry for processes such as metal cleaning, electroplating, and the production of various chemicals.
On the other hand, sodium hydroxide, also known as caustic soda or lye, is a highly caustic base and alkali. It is a white solid, readily soluble in water, forming a strongly alkaline solution. Sodium hydroxide is a key substance in many industries, including pulp and paper manufacturing, soap and detergent production, and as a powerful cleaning agent.
The HCl-NaOH Reaction: A Neutralization Process

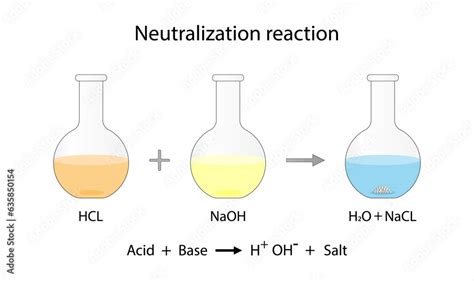
When hydrochloric acid and sodium hydroxide are combined, they undergo a neutralization reaction, a fundamental chemical process where an acid and a base react to form a salt and water.
The equation for this reaction is straightforward: HCl + NaOH → NaCl + H2O. In this reaction, the hydrogen ion (H+) from HCl combines with the hydroxide ion (OH-) from NaOH to form water (H2O), and the sodium ion (Na+) from NaOH combines with the chloride ion (Cl-) from HCl to form sodium chloride (NaCl), a common table salt.
Key Characteristics of the Reaction
- Exothermic Nature: The HCl-NaOH reaction is highly exothermic, releasing a significant amount of heat energy. This property makes it useful in certain industrial processes where heat is required.
- Rapid Reaction: The reaction between HCl and NaOH is typically fast and vigorous, especially when the solutions are concentrated. This rapid reaction rate can be beneficial for certain applications but may also pose safety concerns if not handled properly.
- Neutral pH Result: As a neutralization reaction, the end product of HCl and NaOH has a pH of 7, indicating a neutral solution. This is a significant outcome, as it can be used to neutralize overly acidic or basic solutions, making it a vital process in pH control.
Real-World Applications of the HCl-NaOH Reaction
The HCl-NaOH reaction finds extensive use in various industries and applications, some of which are highlighted below.
1. Chemical Manufacturing
In the chemical industry, the HCl-NaOH reaction is used to produce various salts, such as sodium chloride (NaCl), which has numerous applications. For instance, it is used in the manufacture of dyes, detergents, and even as a food additive.
2. Water Treatment
This reaction is crucial in water treatment processes. By adding HCl and NaOH to water, the pH can be adjusted to ensure it is within safe limits for consumption or industrial use. This is particularly important in processes like water softening and waste water treatment.
3. Food Industry
The food industry also benefits from the HCl-NaOH reaction. Sodium chloride, produced from this reaction, is a common food preservative and flavor enhancer. Additionally, the pH control capabilities of this reaction are vital in processes like pickling and fermentation.
4. Laboratory Applications
In laboratories, the HCl-NaOH reaction is often used for pH calibration and adjustment. It is a reliable and controlled way to prepare solutions of known pH, which is essential for various chemical and biological experiments.
5. Industrial Cleaning
The highly exothermic nature of the HCl-NaOH reaction makes it useful in industrial cleaning processes. The reaction can generate heat, which can help break down and remove stubborn deposits and residues, particularly in applications like oil rig cleaning and industrial equipment maintenance.
Safety Considerations
While the HCl-NaOH reaction has numerous benefits, it is crucial to handle these substances with care due to their corrosive nature. Proper safety equipment, including gloves, goggles, and lab coats, should always be worn when working with these chemicals. Additionally, the reaction should be conducted in a well-ventilated area to prevent the buildup of harmful gases.
Future Implications and Research

The HCl-NaOH reaction is a well-understood process, but ongoing research focuses on optimizing its efficiency and safety. For instance, scientists are exploring ways to minimize the heat generation during the reaction, which could lead to safer and more controlled processes. Additionally, research is being conducted on alternative methods for pH control, which may reduce the reliance on this specific reaction in certain applications.
Conclusion
The reaction between hydrochloric acid and sodium hydroxide is a fundamental chemical process with a wide range of applications. Its exothermic nature, rapid reaction rate, and pH neutralization capabilities make it a valuable tool in various industries. As research continues, we can expect to see further advancements in understanding and utilizing this reaction, contributing to safer and more efficient chemical processes.
What is the main product of the HCl-NaOH reaction?
+The primary product of the HCl-NaOH reaction is sodium chloride (NaCl), commonly known as table salt. This salt is formed when the hydrogen ion (H+) from HCl combines with the hydroxide ion (OH-) from NaOH to produce water (H2O), and the remaining ions (Na+ and Cl-) form NaCl.
Is the HCl-NaOH reaction reversible?
+No, the HCl-NaOH reaction is not reversible under normal conditions. This is because the reaction involves the complete transfer of ions to form a salt and water. In a reversible reaction, the products can break down to reform the reactants, but this is not the case with HCl and NaOH.
What are some safety precautions when handling HCl and NaOH?
+When working with HCl and NaOH, it’s crucial to wear personal protective equipment (PPE) such as gloves, goggles, and a lab coat. These substances are highly corrosive and can cause severe burns. Ensure you are in a well-ventilated area to avoid inhaling harmful fumes, and always keep a neutralizing agent nearby in case of spills.



