Unveiling the Secrets: Potassium's Valence Electrons
Potassium, a versatile and essential element in the periodic table, holds a unique position due to its intriguing electron configuration. Its valence electrons, the outermost electrons that participate in chemical bonding, play a crucial role in determining potassium's reactivity and its behavior in various chemical processes. Unraveling the secrets of potassium's valence electrons provides a fascinating glimpse into the fundamental principles of chemistry and the building blocks of matter.
Understanding Potassium’s Electron Configuration
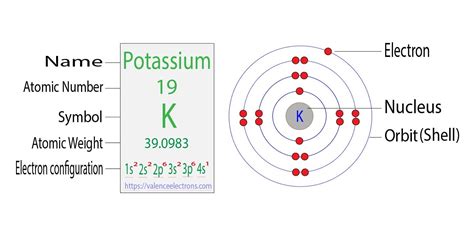
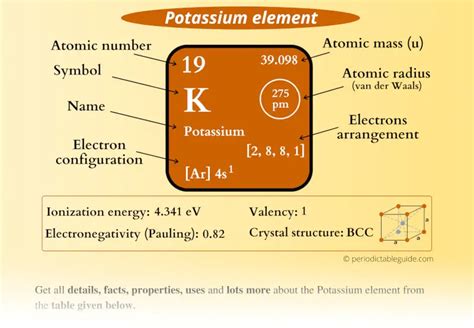
Potassium, represented by the symbol K and positioned in group 1 (alkali metals) and period 4 of the periodic table, boasts an atomic number of 19. This means that a neutral potassium atom possesses 19 protons in its nucleus and, consequently, 19 electrons to maintain electrical neutrality.
The distribution of these electrons across different energy levels, or shells, follows a specific pattern. The first shell, closest to the nucleus, can accommodate up to 2 electrons. The second shell can hold up to 8 electrons, and the third shell, in the case of potassium, is the outermost and is capable of accommodating up to 18 electrons.
In potassium's case, the electron configuration is [Ar] 4s^1. This notation indicates that the first three shells are filled, as denoted by the presence of argon's electron configuration ([Ar]), and the outermost, or valence, shell contains a single electron in the 4s orbital.
The Significance of Valence Electrons
Valence electrons are of paramount importance in chemical bonding and reactivity. These outermost electrons are the primary participants in the formation of chemical bonds, whether through ionic or covalent interactions. The number and arrangement of valence electrons dictate an element’s chemical behavior and its propensity to form bonds with other elements.
| Element | Atomic Number | Electron Configuration | Valence Electrons |
|---|---|---|---|
| Potassium | 19 | [Ar] 4s^1 | 1 |
| Sodium | 11 | [Ne] 3s^1 | 1 |
| Lithium | 3 | [He] 2s^1 | 1 |
As seen in the table above, potassium, sodium, and lithium, all alkali metals, share a common trait: they have just one valence electron in their outermost shell. This singular valence electron makes these elements highly reactive and prone to losing it to achieve a stable electron configuration akin to that of the noble gas in the preceding period.
The Reactivity of Potassium’s Valence Electron
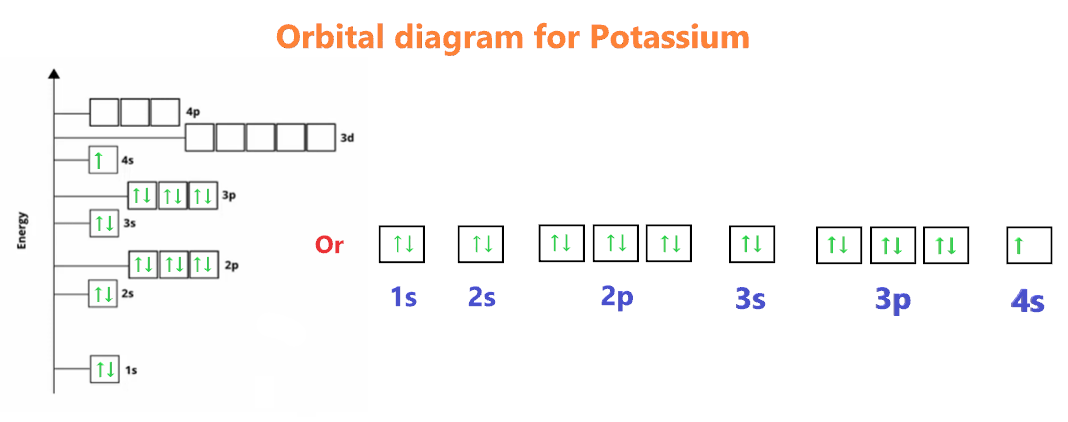
Potassium’s solitary valence electron resides in a relatively high-energy 4s orbital, which means it is relatively easy for potassium to lose this electron. When potassium encounters other elements or compounds, its tendency to lose this electron becomes apparent, leading to the formation of various chemical compounds.
Ionic Bonding
In the realm of ionic bonding, potassium readily donates its valence electron to elements with a high electronegativity, such as halogens (e.g., chlorine, fluorine). This donation results in the formation of ionic compounds. For instance, when potassium reacts with chlorine, it forms potassium chloride (KCl), a common salt.
The chemical equation for this reaction is as follows:
2K + Cl2 → 2KCl
In this reaction, potassium loses its single valence electron to each chlorine atom, resulting in the formation of a potassium ion (K+) and a chloride ion (Cl-). The attraction between these oppositely charged ions gives rise to the ionic bond in potassium chloride.
Covalent Bonding
While potassium primarily forms ionic bonds due to its strong tendency to lose electrons, it can also participate in covalent bonding under specific conditions. In covalent bonding, potassium shares its valence electron with another atom, typically a non-metal with a higher electronegativity.
For instance, when potassium reacts with oxygen, it can form potassium oxide (K2O). In this compound, potassium shares its valence electron with oxygen, forming a covalent bond. The chemical equation for this reaction is:
2K + O2 → 2K2O
In this reaction, each potassium atom donates its single valence electron to an oxygen atom, resulting in the formation of a potassium ion (K+) and an oxide ion (O2-). The covalent bond between these ions stabilizes the compound.
The Impact of Potassium’s Valence Electron on Chemical Behavior
Potassium’s valence electron plays a pivotal role in determining its chemical behavior and reactivity. Its solitary status in the outermost shell makes potassium highly reactive, leading to its propensity to form ionic compounds with a wide range of elements.
Reactivity with Water
One of the most visually striking and dangerous demonstrations of potassium’s reactivity is its interaction with water. When potassium is exposed to water, it undergoes a vigorous reaction that releases hydrogen gas and forms potassium hydroxide (KOH), a strong base.
The chemical equation for this reaction is:
2K + 2H2O → 2KOH + H2
This reaction is highly exothermic, releasing a substantial amount of heat and often resulting in a violent reaction, as the hydrogen gas produced can ignite upon contact with air.
Reactivity with Other Elements
Potassium’s reactivity extends beyond water. It readily reacts with a wide array of elements, forming various compounds. For instance, potassium reacts with sulfur to form potassium sulfide (K2S), and with hydrogen to form potassium hydride (KH), showcasing its versatility in forming compounds with different elements.
Applications and Significance of Potassium Compounds
The compounds formed by potassium, particularly those with non-metals, have diverse applications and play crucial roles in various industries and everyday life.
Potassium Chloride (KCl)
Potassium chloride is a common salt with a wide range of uses. It is used as a fertilizer in agriculture due to its high potassium content, which is essential for plant growth. Additionally, KCl finds applications in medicine, as it serves as a source of potassium ions for various physiological functions.
Potassium Carbonate (K2CO3)
Potassium carbonate, also known as potash, is another important potassium compound. It is used in the manufacture of glass, soap, and various chemical processes. Potash is also an essential ingredient in the production of certain types of fertilizers.
Potassium Hydroxide (KOH)
Potassium hydroxide, often referred to as caustic potash, is a strong base with a wide range of industrial applications. It is used in the production of soaps, detergents, and various chemical processes. KOH is also employed in the manufacture of potassium-based batteries and in the chemical synthesis of other compounds.
Exploring the Electronegativity Trend
Potassium’s position in the periodic table, particularly in group 1, is indicative of its low electronegativity. As one moves down the group, from lithium to sodium to potassium, the electronegativity decreases. This trend is a result of the increasing atomic radius, which causes the valence electrons to be further away from the nucleus and less tightly bound.
The decrease in electronegativity has significant implications for potassium's chemical behavior. It becomes more prone to losing its valence electron, making it a powerful reducing agent. This trend is observed across the entire periodic table and is a fundamental concept in understanding the reactivity of elements.
Future Prospects and Research
The study of potassium’s valence electrons and their role in chemical bonding and reactivity is an ongoing area of research. Scientists continue to explore the unique properties of potassium and its compounds, seeking to unlock new applications and a deeper understanding of the fundamental principles of chemistry.
One area of focus is the development of new potassium-based materials with tailored properties. By manipulating the electron configuration and bonding patterns of potassium, researchers aim to create materials with specific electrical, optical, or magnetic characteristics. These materials could find applications in electronics, energy storage, and various other technologies.
Potassium-Based Batteries
Potassium-based batteries are an emerging field of research, driven by the need for sustainable and efficient energy storage solutions. Unlike traditional lithium-ion batteries, potassium-ion batteries offer the potential for lower cost and improved safety due to the abundance and non-toxic nature of potassium.
Researchers are exploring various aspects of potassium-ion batteries, including the development of suitable electrode materials, electrolytes, and battery architectures. The unique electron configuration of potassium, with its single valence electron, makes it a promising candidate for high-performance energy storage systems.
Conclusion
Potassium’s valence electrons, with their singular presence in the outermost shell, unlock a world of chemical possibilities. From its highly reactive nature, leading to the formation of a multitude of compounds, to its role in essential processes like photosynthesis, potassium is a cornerstone element in the periodic table.
By unraveling the secrets of potassium's valence electrons, scientists and researchers continue to push the boundaries of our understanding of chemistry. This knowledge not only enhances our grasp of the fundamental principles of matter but also paves the way for innovative applications and technologies that can shape our future.
How does potassium’s valence electron configuration affect its reactivity?
+
Potassium’s valence electron configuration, with a single electron in the outermost shell, makes it highly reactive. This electron is relatively easy to lose, allowing potassium to readily form ionic compounds with elements of higher electronegativity.
What are some common compounds formed by potassium?
+
Potassium forms a variety of compounds, including potassium chloride (KCl), potassium carbonate (K2CO3), and potassium hydroxide (KOH). These compounds have diverse applications in agriculture, medicine, and industry.
How does potassium’s reactivity compare to other alkali metals?
+
Potassium is highly reactive, similar to other alkali metals like sodium and lithium. All alkali metals have a single valence electron, making them prone to losing this electron and forming ionic compounds. However, potassium’s larger atomic radius and lower electronegativity make it even more reactive than its lighter counterparts.



