Pi Bonds: A Simple Guide
The world of chemistry is a fascinating one, where complex molecular interactions and structures are governed by intricate bonds. Among these bonds, pi bonds hold a unique place, contributing to the richness and diversity of chemical compounds. In this guide, we will delve into the essence of pi bonds, exploring their nature, formation, and significance in the realm of chemistry.
Unraveling the Nature of Pi Bonds
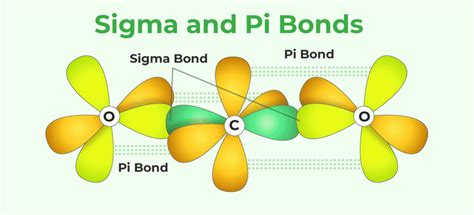
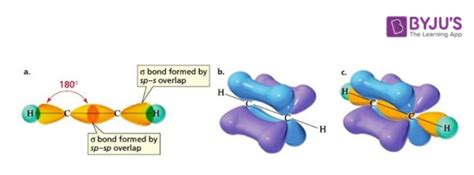
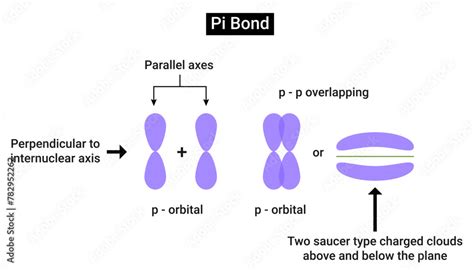
Pi bonds, often denoted as π bonds, are a type of covalent bond formed between atoms, specifically involving the lateral overlap of atomic orbitals. Unlike their counterpart, sigma (σ) bonds, which are formed by the head-on overlap of orbitals, pi bonds involve a side-by-side alignment. This distinct orientation gives rise to their unique characteristics and behaviors.
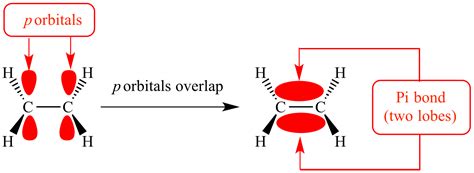
At the heart of pi bond formation is the interaction between two atomic orbitals, typically p-orbitals, but sometimes involving other types of orbitals as well. These orbitals, with their dumbbell-like shape, are oriented perpendicular to the internuclear axis, creating a sideways overlap that results in the formation of the pi bond. The shared electrons within this bond are responsible for the strong attractive forces that hold the atoms together.
The Role of Orbitals in Pi Bond Formation
To understand pi bonds, one must delve into the intricacies of atomic orbitals. Orbitals, as described by quantum mechanics, are regions in space where electrons are most likely to be found. In the case of pi bonds, the key players are the p-orbitals, which possess two lobes extending in opposite directions. When two p-orbitals align side by side, they create a region of electron density above and below the internuclear axis, leading to the formation of the pi bond.
While p-orbitals are the primary contributors to pi bonds, other orbitals can also participate. For instance, in certain molecules, d-orbitals can engage in pi bonding, expanding the possibilities for complex molecular structures. The versatility of orbitals in pi bond formation adds to the richness and diversity of the chemical world.
| Molecule | Pi Bonds |
|---|---|
| Ethene (C2H4) | 1 |
| Benzene (C6H6) | 3 |
| Ethylene Oxide (C2H4O) | 2 |
The Strength and Stability of Pi Bonds
Pi bonds, despite their lateral orientation, exhibit remarkable strength and stability. The shared electrons within the pi bond are held firmly by the attractive forces between the nuclei and the electron cloud. This stability arises from the efficient distribution of electron density, which minimizes the electrostatic repulsion between electrons and maximizes the attractive forces.
However, the very nature of pi bonds also makes them susceptible to certain interactions and disruptions. For instance, pi bonds can be broken or distorted by external factors such as heat or the presence of specific reagents. This susceptibility to change contributes to the reactivity and transformation potential of molecules containing pi bonds.
The Impact of Pi Bond Stability on Molecular Properties
The stability of pi bonds plays a crucial role in determining the physical and chemical properties of molecules. Molecules with multiple pi bonds, for instance, often exhibit higher boiling points and lower solubility in non-polar solvents. This is due to the stronger intermolecular forces resulting from the presence of multiple pi bonds.
Additionally, the stability of pi bonds influences the reactivity of molecules. Molecules with easily breakable pi bonds tend to be more reactive, as the disruption of these bonds can lead to the formation of new bonds and the creation of new compounds. This reactivity is a key factor in many chemical reactions and processes, making pi bonds a central focus in the study of chemistry.
Pi Bonds in Action: Real-World Examples
Pi bonds are not merely theoretical concepts; they are the building blocks of countless molecules and compounds that surround us. From the natural world to industrial processes, pi bonds play a vital role in shaping the chemical landscape.
In the realm of organic chemistry, pi bonds are prevalent in hydrocarbons and aromatic compounds. For instance, ethene, a simple hydrocarbon, contains a single pi bond between its carbon atoms, contributing to its chemical behavior and reactivity. Similarly, benzene, with its six carbon atoms and alternating single and double bonds, showcases the intricate dance of sigma and pi bonds that give rise to its unique properties.
Pi bonds also extend beyond organic chemistry, finding applications in various fields. In materials science, for example, pi bonds are crucial in the formation of conductive polymers, where the delocalized pi electrons contribute to the material's electrical conductivity. Additionally, in biological systems, pi bonds are essential in the structure and function of DNA and RNA, where they play a critical role in maintaining the stability of these genetic molecules.
The Versatility of Pi Bonds in Chemistry
Pi bonds showcase their versatility in a myriad of chemical reactions and processes. In addition to their role in stabilizing molecular structures, pi bonds are actively involved in various types of reactions, such as addition reactions and electrophilic aromatic substitution.
Addition reactions, where a pi bond is broken and new bonds are formed, are common in the synthesis of many organic compounds. For instance, the addition of hydrogen to an alkene, a molecule containing a pi bond, results in the formation of an alkane, a saturated hydrocarbon. This simple reaction highlights the transformative power of pi bonds and their role in chemical synthesis.
Furthermore, pi bonds are pivotal in the field of organic synthesis, where chemists harness their reactivity to create complex molecules. The ability to manipulate and control pi bonds allows for the precise construction of molecular structures, leading to the development of pharmaceuticals, materials, and numerous other compounds that impact our daily lives.
Exploring the Future of Pi Bond Research
While pi bonds have been studied extensively, there is still much to uncover and explore. Ongoing research in the field of chemistry continues to delve deeper into the intricacies of pi bonding, aiming to understand its role in complex molecular systems and its potential applications.
One area of focus is the study of pi bonds in unconventional molecular systems, such as non-carbon-based compounds. Researchers are investigating the formation and behavior of pi bonds in molecules containing elements beyond carbon, exploring the boundaries of traditional chemistry and expanding our understanding of molecular interactions.
Potential Applications and Innovations
The future of pi bond research holds the promise of exciting innovations and applications. For instance, the study of pi bonds in organic light-emitting diodes (OLEDs) is paving the way for more efficient and sustainable lighting technologies. By understanding and manipulating pi bonds in these compounds, researchers aim to enhance the efficiency and longevity of OLEDs, offering a greener alternative to traditional lighting.
Additionally, pi bonds are at the forefront of research in the field of organic electronics. The development of organic semiconductors, which rely on the conductive properties of pi bonds, has the potential to revolutionize electronics, offering flexible, lightweight, and cost-effective alternatives to traditional silicon-based devices. The ongoing exploration of pi bonds in this context holds immense promise for the future of technology.
Conclusion
Pi bonds, with their unique lateral orientation and strong electron sharing, are a cornerstone of chemical bonding. From the fundamental principles of quantum mechanics to the intricate structures of molecules, pi bonds play a vital role in shaping the chemical world. As research continues to unravel the mysteries of pi bonding, we can expect new discoveries and applications that will further our understanding of chemistry and its impact on our lives.
Whether in the synthesis of new compounds, the study of molecular structures, or the development of cutting-edge technologies, pi bonds remain a fundamental and fascinating aspect of chemistry. This guide has provided a glimpse into the world of pi bonds, offering a deeper understanding of their nature, formation, and significance. As we continue to explore the wonders of chemistry, pi bonds will undoubtedly remain a key focus, guiding us towards new frontiers of scientific discovery.
How do pi bonds differ from sigma bonds?
+Pi bonds differ from sigma bonds in their orientation and formation. Pi bonds involve the lateral overlap of atomic orbitals, typically p-orbitals, creating a region of electron density above and below the internuclear axis. In contrast, sigma bonds are formed by the head-on overlap of orbitals, resulting in a shared electron pair along the internuclear axis.
Are pi bonds always present in molecules?
+No, pi bonds are not always present in molecules. Their presence depends on the molecular structure and the specific arrangement of atomic orbitals. Simple molecules like methane (CH4) do not have pi bonds, as all the bonds are sigma bonds. However, in more complex molecules, such as alkenes and aromatic compounds, pi bonds are common and play a crucial role in their properties and reactivity.
Can pi bonds be broken and reformed during chemical reactions?
+Yes, pi bonds can be broken and reformed during chemical reactions. Many reactions, such as addition reactions and electrophilic aromatic substitution, involve the disruption of pi bonds and the formation of new bonds. This dynamic behavior of pi bonds allows for the transformation of molecules and the creation of new compounds.



