5 Secrets to Perfect Liquid-Liquid Dissolutions

In the world of chemistry, mastering the art of liquid-liquid dissolutions is crucial for various applications, from pharmaceutical formulations to environmental science. This comprehensive guide delves into five pivotal strategies that ensure optimal results, offering an insightful perspective on this intricate process.
1. Understanding Solubility Parameters
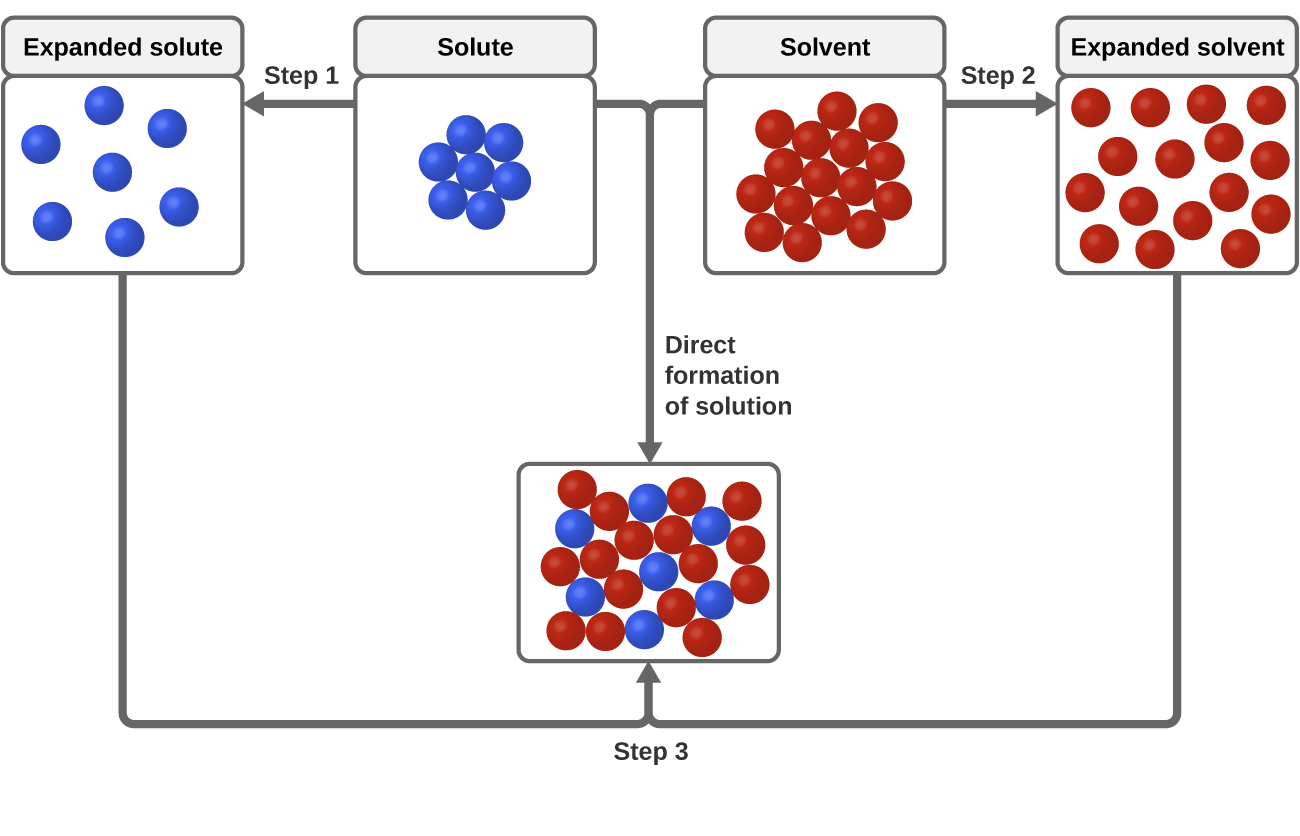
The cornerstone of successful liquid-liquid dissolutions lies in comprehending solubility parameters. These parameters, often denoted by the symbol δ, encapsulate key factors such as polarity, hydrogen bonding, and dispersion forces. The proximity of these parameters between the solvent and solute is pivotal in achieving efficient dissolutions.
For instance, consider the dissolution of ibuprofen, a non-steroidal anti-inflammatory drug, in a mixture of ethanol and water. Ibuprofen, with its semi-polar nature, exhibits a solubility parameter that aligns well with ethanol's, facilitating dissolution. Conversely, its parameter mismatch with water highlights the challenge of complete dissolution in this solvent.
To enhance solubility, scientists often manipulate the solvent composition, leveraging the concept of mixing rules to predict the solubility parameter of a solvent mixture. This strategic approach ensures that the chosen solvent system aligns optimally with the solute's characteristics.
Exploring Solubility Curves
Visual aids, such as solubility curves, offer invaluable insights into the solubility behavior of substances. These curves plot the solubility of a solute against varying temperatures and solvent compositions. By referencing such curves, chemists can identify the ideal conditions for dissolution, ensuring both efficiency and stability.
A practical example involves the dissolution of caffeine, a compound with a unique solubility profile. Caffeine's solubility curve reveals its enhanced solubility at higher temperatures, a phenomenon often leveraged in coffee extraction processes. Understanding such nuances is pivotal for achieving precise dissolutions in various industries.
| Solvent | Solubility Parameter δ (MPa1/2) |
|---|---|
| Ethanol | 18.7 |
| Water | 23.4 |
| Acetone | 20.3 |
2. Mastering the Art of Mixing Techniques

The efficiency of liquid-liquid dissolutions hinges on the selection and implementation of appropriate mixing techniques. These techniques, ranging from simple stirring to advanced ultrasonic methods, play a pivotal role in facilitating intimate contact between solvent and solute molecules.
Stirring and Agitation
Stirring, the most conventional mixing method, is a versatile approach suitable for a wide range of dissolutions. The effectiveness of stirring depends on factors such as the size and shape of the stirring apparatus, the rotation speed, and the duration of the process. For instance, a magnetic stirrer with a Teflon-coated stir bar is often the go-to choice for laboratory-scale dissolutions.
To optimize stirring efficiency, chemists often consider the Reynolds number, a dimensionless quantity that characterizes the flow regime. A higher Reynolds number, indicative of turbulent flow, generally enhances mass transfer and dissolution rates.
Ultrasonic Mixing
Ultrasonic mixing, employing high-frequency sound waves, offers a powerful alternative for challenging dissolutions. This technique, also known as sonication, induces cavitation, a process where tiny bubbles form and collapse, generating intense local forces that disrupt solute particles and enhance solubility.
Ultrasonic mixing finds its application in the dissolution of highly viscous or insoluble substances. For example, in the pharmaceutical industry, sonication is used to dissolve polymers or lipophilic drugs that are notoriously difficult to solubilize using conventional methods.
The intensity and duration of ultrasonic mixing are critical parameters. Excessive sonication can lead to unwanted side effects, such as solvent evaporation or degradation of sensitive compounds. Thus, a nuanced understanding of the process is essential for optimal results.
3. Temperature and Pressure Control
Temperature and pressure are potent tools in the chemist’s arsenal for manipulating solubility. These variables influence the kinetic and thermodynamic aspects of dissolution, offering a strategic approach to achieving desired outcomes.
Leveraging Temperature Effects
Temperature plays a dual role in liquid-liquid dissolutions. On the one hand, it influences the kinetic energy of molecules, affecting their ability to mix and dissolve. On the other hand, it impacts the thermodynamics of the system, altering the equilibrium solubility of the solute.
Increasing temperature generally enhances dissolution rates by providing the necessary energy for solute molecules to overcome the activation energy barrier. This principle is exemplified in the extraction of essential oils from plants, where heat facilitates the release of volatile compounds.
Conversely, temperature manipulation can also be used to promote phase separation, a crucial step in many separation processes. By carefully controlling temperature, chemists can induce the precipitation of desired compounds, facilitating their subsequent isolation.
Exploring Pressure Effects
Pressure, particularly in the context of supercritical fluids, offers a unique approach to dissolution. Supercritical fluids, such as supercritical carbon dioxide, exhibit properties of both gases and liquids, making them versatile solvents. By manipulating pressure, scientists can fine-tune the solubility behavior of these fluids, enabling precise dissolutions.
Supercritical fluid extraction (SFE) has gained prominence in the food and pharmaceutical industries due to its ability to selectively extract desired compounds while minimizing thermal degradation. The flexibility in pressure control allows for tailored extractions, catering to specific applications.
4. Utilizing Solubilizing Agents
Solubilizing agents, also known as cosolvents or surfactants, are invaluable tools for enhancing the solubility of challenging compounds. These agents, through various mechanisms, modify the solvation environment, making it more favorable for dissolution.
The Role of Cosolvents
Cosolvents, when added to a solvent system, can significantly alter its solubility properties. This effect is particularly pronounced when the cosolvent has a solubility parameter that bridges the gap between the solvent and the solute. By acting as a bridge, the cosolvent facilitates the dissolution of the solute.
A classic example is the use of dimethyl sulfoxide (DMSO) as a cosolvent. DMSO, with its unique ability to dissolve a wide range of compounds, is often employed to enhance the solubility of otherwise insoluble drugs in pharmaceutical formulations.
Surfactants: Nature’s Solubilizers
Surfactants, or surface-active agents, are a diverse class of compounds that reduce the surface tension between phases, thereby enhancing the solubility of solutes. These compounds, often derived from natural sources, are particularly effective in the dissolution of amphiphilic molecules, such as lipids and proteins.
One notable example is the use of bile salts, a class of surfactants produced in the liver, for the dissolution of dietary fats during digestion. Bile salts, with their unique structure, facilitate the emulsification and solubilization of fats, enabling their absorption in the small intestine.
5. Optimization through Experimental Design

Achieving perfect liquid-liquid dissolutions often requires a systematic approach, involving the application of experimental design principles. These principles guide the selection of variables, the design of experiments, and the interpretation of results, ensuring an efficient and data-driven process.
Design of Experiments (DoE)
DoE, a powerful statistical tool, allows chemists to explore the effects of multiple variables simultaneously. By employing techniques such as full factorial or fractional factorial designs, researchers can efficiently identify the optimal conditions for dissolution.
For instance, in a study investigating the dissolution of a novel drug, a DoE approach might involve varying factors such as solvent composition, temperature, and agitation speed. By analyzing the results, researchers can pinpoint the combination of factors that yields the highest dissolution efficiency.
Response Surface Methodology (RSM)
RSM takes experimental design a step further by incorporating mathematical models to predict the response of a system. This methodology, often coupled with statistical analysis, provides a deeper understanding of the relationship between variables and the response, allowing for precise optimization.
In the context of liquid-liquid dissolutions, RSM can be used to model the effect of temperature, pressure, and solvent composition on the solubility of a solute. By fitting the data to a response surface, researchers can identify the "sweet spot" where the desired dissolution behavior is achieved.
Conclusion
Mastering liquid-liquid dissolutions is a complex yet rewarding endeavor, offering a deep understanding of the interplay between solvents, solutes, and process variables. By harnessing the power of solubility parameters, mixing techniques, temperature and pressure control, solubilizing agents, and experimental design, chemists can achieve unparalleled precision and efficiency in their dissolutions.
As the field of chemistry continues to advance, the strategies outlined in this guide will serve as a solid foundation for researchers, paving the way for new discoveries and innovations in various industries. The art of dissolution, when perfected, opens doors to a world of possibilities, from creating life-saving medications to unlocking the secrets of natural products.
What are the key factors influencing the solubility of a solute in a liquid-liquid system?
+
The solubility of a solute in a liquid-liquid system is primarily influenced by the solute’s molecular structure, the solvent’s polarity, and the temperature and pressure of the system. The solubility parameter, which quantifies these factors, plays a crucial role in determining whether a solute will dissolve in a given solvent.
How can I enhance the solubility of a poorly soluble compound?
+
Enhancing solubility can be achieved through various strategies, including the use of cosolvents or surfactants, adjusting temperature and pressure conditions, and employing advanced mixing techniques such as ultrasonic mixing. Each approach should be tailored to the specific compound and solvent system.
What is the significance of mixing techniques in liquid-liquid dissolutions?
+
Mixing techniques are crucial for ensuring effective contact between the solvent and solute molecules. They influence the rate and extent of dissolution by promoting intimate mixing and facilitating mass transfer. The choice of mixing technique depends on factors such as the nature of the solute, the desired dissolution rate, and the scale of the operation.
Can you provide an example of how temperature control can be used to manipulate solubility?
+
Certainly! One example is the extraction of caffeine from coffee beans. Caffeine is more soluble at higher temperatures, so increasing the temperature during the extraction process can enhance the solubility of caffeine in water, leading to a more efficient extraction.
What are some common applications of liquid-liquid dissolutions in industry and research?
+
Liquid-liquid dissolutions find applications in a wide range of industries, including pharmaceuticals (drug formulation and extraction), food processing (extraction of flavors and nutrients), environmental science (water treatment and pollution control), and chemical synthesis (separation and purification of compounds). They are also essential in research, particularly in the development of new materials and the study of complex chemical reactions.