5 Tips for Lewis Structure Mastery
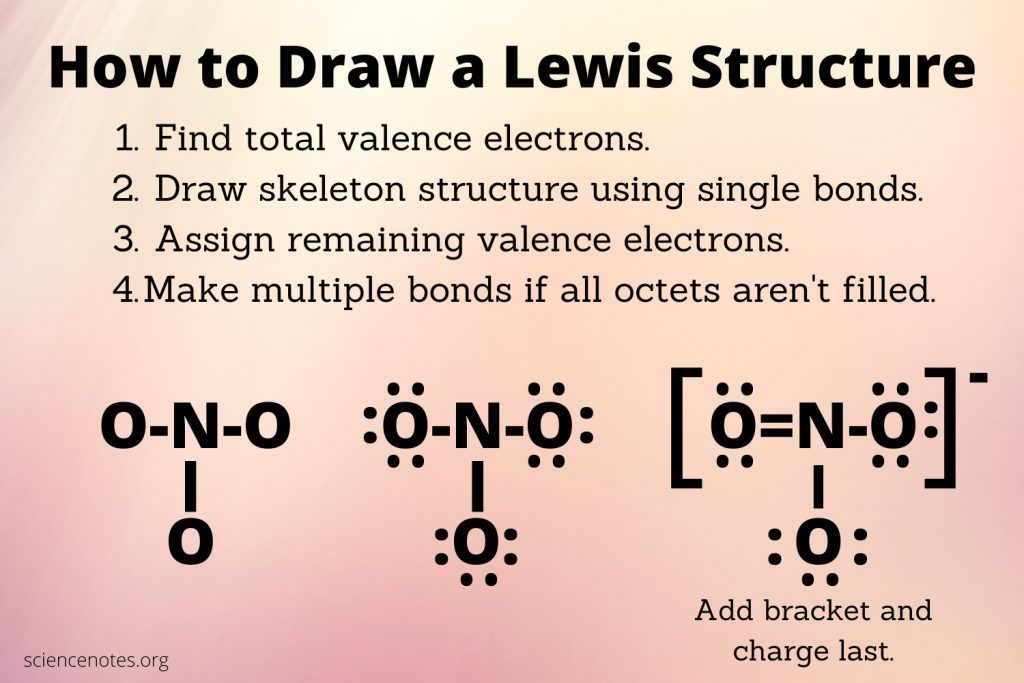
Mastering the art of drawing Lewis structures is an essential skill for any chemist or student delving into the world of molecular chemistry. Lewis structures, named after Gilbert N. Lewis, provide a visual representation of the distribution of electrons in molecules and ions, offering insights into bonding, stability, and reactivity. In this comprehensive guide, we will explore five expert tips to help you become a pro at drawing Lewis structures and unlock the secrets of molecular geometry and behavior.
1. Understand the Basics of Electron Configuration
The foundation of Lewis structure drawing lies in a solid grasp of electron configuration. Before you begin, ensure you are familiar with the Aufbau principle, which dictates how electrons fill atomic orbitals. Understand the concept of atomic shells, subshells, and orbitals, and how electrons are distributed in different elements. This knowledge forms the basis for predicting the number of valence electrons and the bonding behavior of atoms.
For instance, consider the element carbon. Carbon has an electron configuration of 1s² 2s² 2p², with four valence electrons in its outermost 2s and 2p orbitals. This understanding is crucial when determining the number of electrons available for bonding and non-bonding electron pairs.
Valence Electrons and Lewis Structures
Valence electrons play a pivotal role in Lewis structures. These are the electrons in the outermost energy level of an atom, which are typically involved in bonding. When drawing Lewis structures, it’s essential to identify the valence electrons of each atom in the molecule. Here’s a simple breakdown:
| Element | Atomic Number | Valence Electrons |
|---|---|---|
| Hydrogen (H) | 1 | 1 |
| Carbon © | 6 | 4 |
| Oxygen (O) | 8 | 6 |
| Nitrogen (N) | 7 | 5 |
| Sulfur (S) | 16 | 6 |
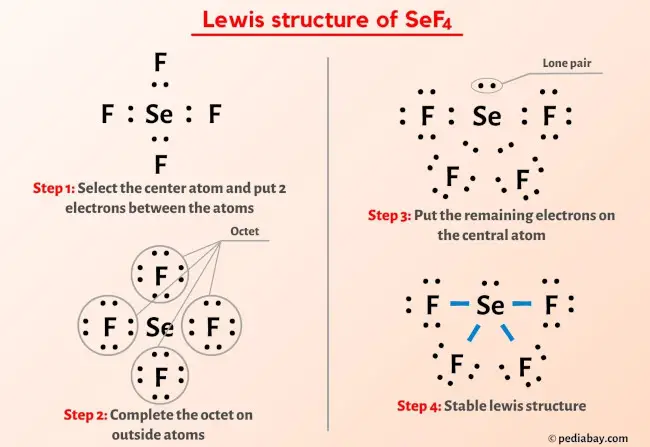
2. Master the Octet Rule
The octet rule is a fundamental principle in Lewis structure drawing. It states that atoms tend to gain, lose, or share electrons to achieve a stable electronic configuration, similar to that of a noble gas. In most cases, this means achieving a total of eight electrons in the outermost shell, forming an “octet.”
For example, when drawing the Lewis structure of methane (CH4), each carbon atom achieves an octet by forming four single bonds with hydrogen atoms. Similarly, in ammonia (NH3), the nitrogen atom achieves an octet by forming three single bonds with hydrogen atoms and having one lone pair of electrons.
Exceptions to the Octet Rule
While the octet rule is a valuable guide, it’s important to recognize that it has exceptions. Elements in the third period and beyond can accommodate more than eight electrons in their valence shells. These exceptions often involve elements in the third row of the periodic table, such as phosphorus (P), sulfur (S), and chlorine (Cl). These elements can expand their valence shells to accommodate more than eight electrons, forming what is known as an “expanded octet.”
For instance, in the Lewis structure of sulfur hexafluoride (SF6), sulfur has 12 electrons in its valence shell, exceeding the octet rule. This expanded octet stability arises from the availability of 3d orbitals in sulfur, which can accommodate additional electrons.
3. Practice with Common Molecules
To become proficient in drawing Lewis structures, practice is key. Start with common molecules and ions, gradually building up your repertoire. Here are a few examples to get you started:
- Water (H2O)
- Ammonia (NH3)
- Methane (CH4)
- Carbon Dioxide (CO2)
- Nitric Acid (HNO3)
As you practice, pay attention to the distribution of electrons, the formation of bonds, and the stability of the resulting structures. This hands-on approach will reinforce your understanding of electron behavior and the principles of Lewis structure drawing.
Real-World Application: Chemical Reactions
Lewis structures are not just theoretical concepts; they have practical applications in understanding chemical reactions. By drawing Lewis structures of reactants and products, you can visualize how electrons are rearranged during a reaction. This visual representation aids in predicting reaction mechanisms and outcomes.
For instance, consider the reaction between hydrogen (H2) and oxygen (O2) to form water (H2O). By comparing the Lewis structures of the reactants and the product, you can observe the transfer of electrons and the formation of covalent bonds.
4. Utilize Lewis Dot Symbols for Ions
When dealing with ions, Lewis dot symbols provide a concise way to represent their electronic structure. Lewis dot symbols show the distribution of electrons in an atom or ion, including any electrons gained or lost during ionization. These symbols are particularly useful when drawing Lewis structures for polyatomic ions.
For example, consider the sulfate ion (SO42-). The Lewis dot symbol for sulfur in this ion would show six valence electrons, while each oxygen atom would have six valence electrons, with two additional electrons from the negative charge. These extra electrons are often represented as dots around the symbol.
Polyatomic Ions: A Complex Yet Manageable Challenge
Polyatomic ions, consisting of multiple atoms bonded together, can initially seem daunting to draw. However, with practice, you’ll find that they follow the same principles as single atoms or simple molecules. The key is to understand the valence electrons of each atom and how they interact to form stable structures.
Take the example of the ammonium ion (NH4+). Despite its complexity, you can draw its Lewis structure by recognizing that nitrogen has five valence electrons and each hydrogen atom has one. By sharing electrons, the nitrogen atom achieves an octet, and each hydrogen atom forms a single bond.
5. Check for Resonance Structures
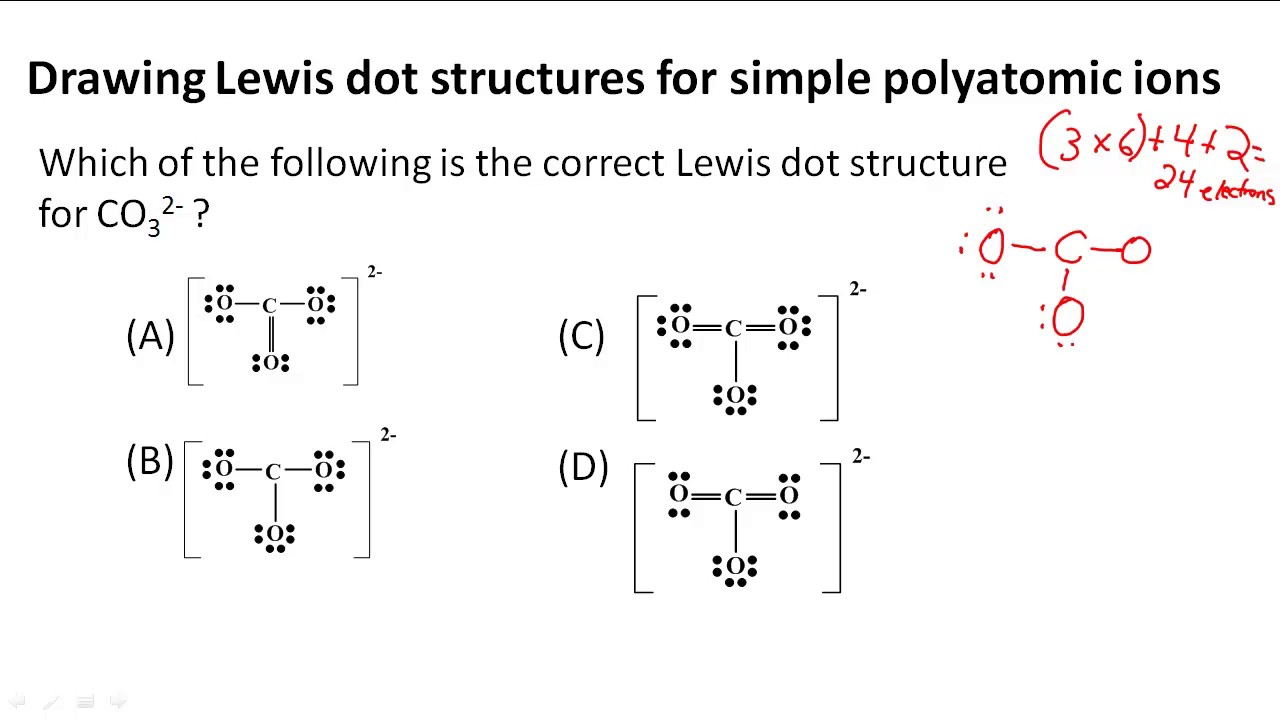
Resonance structures are a unique aspect of Lewis structures, representing different possible arrangements of electrons in a molecule. These structures are particularly relevant when dealing with molecules with multiple bonds or lone pairs. Resonance structures contribute to the overall stability of the molecule, as the delocalization of electrons can lower its energy.
For instance, consider the nitrate ion (NO3–). This ion has three possible resonance structures, each with a different arrangement of double and single bonds. The actual structure is a combination of these resonance forms, resulting in a more stable configuration.
Identifying Resonance Structures: A Step-by-Step Guide
- Identify the molecule or ion with potential for resonance.
- Draw the Lewis structure as you normally would, paying attention to the distribution of electrons.
- Look for regions where electron rearrangement could lead to a more stable structure.
- Create additional Lewis structures by moving electron pairs to create different bonding patterns.
- Ensure that each resonance structure follows the octet rule and has the same total number of electrons.
- Analyze the resonance structures to determine their relative stability and contribution to the overall molecule.
Conclusion: Mastery Through Practice and Understanding
Drawing Lewis structures is a skill that develops with practice and a deep understanding of electron behavior. By mastering the principles outlined in this guide, you’ll be well on your way to becoming a Lewis structure pro. Remember, chemistry is a journey of discovery, and each Lewis structure you draw brings you one step closer to unlocking the secrets of molecular interactions.
FAQ
How do I determine the number of valence electrons for an element?
+
The number of valence electrons for an element can be determined by its position in the periodic table. Elements in the same group (column) typically have the same number of valence electrons. For example, elements in Group 1 (e.g., Hydrogen) have 1 valence electron, while elements in Group 18 (e.g., Helium) have 2 valence electrons.
What is the significance of the octet rule in Lewis structures?
+
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electronic configuration, similar to that of a noble gas. This rule is based on the concept that having 8 valence electrons (an octet) results in a stable, low-energy state. It’s a guiding principle when drawing Lewis structures, helping to predict bonding patterns and molecular stability.
How do I know when to use resonance structures?
+
Resonance structures are used when a single Lewis structure cannot accurately represent the electron distribution in a molecule. This often occurs in molecules with multiple bonds or lone pairs. Resonance structures are drawn to show different arrangements of electrons, each contributing to the overall stability of the molecule.
Can Lewis structures be used to predict molecular geometry?
+
Yes, Lewis structures provide valuable insights into molecular geometry. By analyzing the distribution of electrons and bonds, you can make predictions about the shape and arrangement of atoms in a molecule. This is particularly useful in fields like organic chemistry and molecular modeling.
Are there any software tools that can assist in drawing Lewis structures?
+
Absolutely! There are several software tools available that can aid in drawing Lewis structures. These tools often provide interactive interfaces, allowing you to input molecular formulas and generate accurate Lewis structures. Some popular options include MarvinSketch, ChemDoodle, and ChemDraw.



