Mastering Lewis Dot Structures: 5 Easy Tips
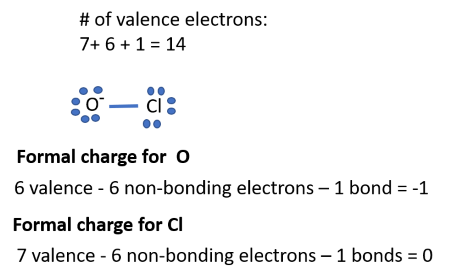
Lewis dot structures, also known as electron dot diagrams or Lewis structures, are a fundamental concept in chemistry, especially in the realm of molecular bonding and understanding the arrangement of electrons in atoms and molecules. These structures provide a visual representation of the distribution of valence electrons, which are the electrons in the outermost energy level of an atom, participating in chemical bonding. In this article, we delve into the world of Lewis dot structures, offering five essential tips to master this crucial skill, ensuring you gain a deeper understanding of chemical compounds and their unique bonding characteristics.
Understanding the Basics of Lewis Dot Structures

Lewis dot structures are an invaluable tool for chemists and students alike, as they offer a simplified yet informative way to represent the electronic structure of atoms and molecules. By following a set of well-defined rules, you can construct accurate Lewis structures, providing insights into the nature of chemical bonds, the distribution of electrons, and the overall stability of a compound.
Tip 1: Know the Octet Rule
The foundation of Lewis dot structures lies in the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration similar to that of a noble gas. In simpler terms, most elements strive to have a full outer energy level, which is usually eight electrons, akin to the noble gases in the same period. This rule is particularly useful when determining the number of valence electrons an atom can contribute to a molecule and its preferred bonding behavior.
| Element | Valence Electrons |
|---|---|
| Carbon (C) | 4 |
| Oxygen (O) | 6 |
| Hydrogen (H) | 1 |

For instance, when drawing the Lewis structure for carbon dioxide (CO2), you'll notice that carbon, with four valence electrons, forms double bonds with two oxygen atoms, each having six valence electrons. This results in a stable molecule where all atoms achieve an octet, fulfilling the octet rule.
Tip 2: Count Valence Electrons Accurately
The first step in constructing a Lewis dot structure is to determine the number of valence electrons for each atom in the molecule. This information is crucial for understanding how atoms will bond and form the molecule’s overall structure. For example, let’s consider the molecule ammonia (NH3). Nitrogen, the central atom, has five valence electrons, while each hydrogen atom contributes one valence electron. This means the total number of valence electrons in ammonia is eight, which allows each atom to achieve the stability of an octet.
| Atom | Valence Electrons |
|---|---|
| Nitrogen (N) | 5 |
| Hydrogen (H) | 1 |
| Total | 8 |
Tip 3: Identify Central and Peripheral Atoms
In many molecules, one type of atom acts as the central atom, forming bonds with other atoms that surround it. The central atom is usually the least electronegative element among the available choices. For instance, in water (H2O), oxygen is the central atom, as it has a higher electronegativity than hydrogen. Understanding which atom will take the central position is crucial for correctly drawing the Lewis structure and determining the overall molecular geometry.
Tip 4: Forming Bonds and Achieving Stability
Once you’ve identified the central atom and determined the number of valence electrons, you can start forming bonds. Atoms will share electrons to achieve a stable electron configuration, often striving for the octet rule. Single, double, or triple bonds may form, depending on the number of electrons needed to reach stability. For example, in carbon dioxide (CO2), carbon forms double bonds with two oxygen atoms, ensuring each atom has a full octet.
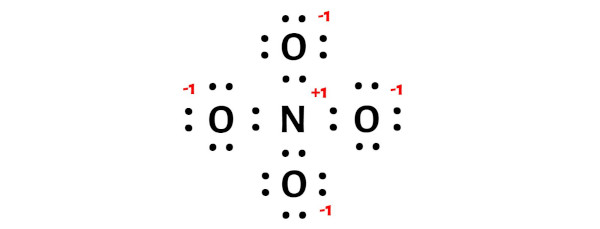
Tip 5: Resonance Structures and Formal Charges
In some cases, a molecule may have multiple valid Lewis structures due to the delocalization of electrons. These are known as resonance structures, and they contribute to the overall stability of the molecule. Additionally, understanding formal charges can help identify the most stable structure. Formal charge considers the number of valence electrons an atom has, the number it uses in bonding, and the number of lone pairs. By minimizing formal charges, you can often identify the most favored Lewis structure.
Applying Lewis Dot Structures: Real-World Examples
Lewis dot structures find application in various fields of chemistry, from organic chemistry to inorganic and even biological systems. Let’s explore a few examples to illustrate their importance.
Organic Chemistry: Benzene (C6H6)
Benzene is a fundamental compound in organic chemistry, featuring a ring structure with alternating single and double bonds. Its Lewis structure reveals a unique bonding pattern where each carbon atom is bonded to two other carbon atoms and one hydrogen atom. This arrangement ensures that all atoms achieve an octet, making benzene a highly stable molecule.
Inorganic Chemistry: Sodium Chloride (NaCl)
Sodium chloride, or table salt, is an excellent example of an ionic compound. In its Lewis structure, sodium, with one valence electron, transfers this electron to chlorine, which has seven valence electrons. This transfer results in a stable ionic bond, with sodium becoming Na+ and chlorine becoming Cl-. The octet rule is satisfied for both atoms, showcasing the versatility of Lewis structures in representing different types of bonds.
Biological Chemistry: Glucose (C6H12O6)
Glucose, a simple sugar, plays a crucial role in biological systems, providing energy for cellular processes. Its Lewis structure reveals a complex network of single and double bonds, with carbon atoms forming the backbone of the molecule. Oxygen atoms, with their higher electronegativity, attract electrons from carbon and hydrogen atoms, creating a stable molecular structure that is vital for life processes.
The Future of Lewis Dot Structures: Advanced Applications
While Lewis dot structures provide a foundational understanding of molecular bonding, they are just the beginning. Advanced concepts, such as molecular orbital theory and quantum chemistry, build upon the principles of Lewis structures to offer a more detailed and nuanced understanding of molecular behavior. These theories delve into the complex interactions of electrons within molecules, providing insights into molecular spectroscopy, reactivity, and even the design of new materials.
Furthermore, computational chemistry and molecular modeling software have revolutionized the study of chemical compounds. These tools allow chemists to simulate and visualize molecular structures and properties, offering a deeper understanding of how molecules interact and react under various conditions. By combining the simplicity of Lewis dot structures with the power of advanced computational methods, chemists can make significant advancements in drug design, material science, and even environmental chemistry.
What are some common challenges when drawing Lewis dot structures, and how can they be overcome?
+
Drawing Lewis dot structures can sometimes be challenging, especially when dealing with complex molecules or compounds that don’t readily follow the octet rule. Here are some common challenges and strategies to overcome them:
- Exceptional Molecules: Some molecules, like sulfur compounds, don’t always follow the octet rule. In such cases, aim for the most stable structure by minimizing formal charges and ensuring that each atom has a relatively stable electron configuration.
- Resonance Structures: When a molecule has multiple valid Lewis structures due to resonance, it’s important to consider all possibilities. Draw all reasonable resonance structures and evaluate their stability based on formal charges and electron distribution.
- Large Molecules: Complex molecules with many atoms can be daunting. Break the molecule down into smaller fragments, drawing the Lewis structures for each fragment, and then combine them to form the overall molecule. This step-by-step approach can make the process more manageable.
- Unusual Bonding: Certain compounds, like boron hydrides, have unusual bonding patterns. In these cases, consult reliable references or seek guidance from experts to understand the unique bonding characteristics and draw accurate Lewis structures.
- Practice and Perseverance: Drawing Lewis dot structures becomes easier with practice. Work through a variety of examples, ranging from simple molecules to more complex ones, to develop your skills and understanding of molecular bonding.
How do Lewis dot structures help in predicting molecular geometry and bond angles?
+
Lewis dot structures provide valuable insights into molecular geometry and bond angles by revealing the arrangement of atoms and the types of bonds formed. Here’s how they contribute to predicting these molecular properties:
- Central Atom Identification: Lewis structures help identify the central atom in a molecule. This is crucial because the central atom’s position and bonding patterns influence the overall molecular geometry.
- Bond Types: By indicating the types of bonds (single, double, or triple), Lewis structures provide information about the lengths and strengths of these bonds. This is essential for understanding bond angles and molecular geometry.
- Valence Shell Electron Pair Repulsion (VSEPR) Theory: VSEPR theory is often used in conjunction with Lewis structures to predict molecular geometry. It states that electron pairs (both bonding and non-bonding) repel each other, influencing the molecular shape. Lewis structures aid in counting these electron pairs and applying VSEPR principles to predict geometry.
- Molecular Modeling: Advanced computational tools can simulate and visualize molecular structures based on Lewis dot representations. These models provide detailed insights into bond lengths, angles, and molecular shapes, allowing for precise predictions and comparisons with experimental data.
Are there any software tools or resources that can assist in drawing Lewis dot structures accurately?
+
Absolutely! Several software tools and online resources are available to assist in drawing Lewis dot structures accurately. Here are a few options:
- ChemDoodle: ChemDoodle is a versatile chemistry software suite that offers a user-friendly interface for drawing and editing molecular structures, including Lewis dot structures. It provides tools for adding and editing atoms, bonds, and electron pairs, making it a powerful resource for creating accurate representations.
- MolView: MolView is an online chemical structure drawing tool that allows users to create and manipulate molecular structures, including Lewis dot diagrams. It provides a range of features, such as adding atoms, bonds, and charges, and supports various file formats for saving and sharing structures.
- ChemDraw: ChemDraw is a widely used professional chemistry software that offers advanced tools for drawing and editing chemical structures. While it may require a steeper learning curve, it provides precise control over molecular representations, making it an excellent choice for creating high-quality Lewis dot structures.
- Online Tutorials and Guides: Numerous online tutorials and guides are available, offering step-by-step instructions and examples for drawing Lewis dot structures. These resources can be invaluable for beginners and advanced users alike, providing clear guidance and tips for accurate structure drawing.



