Is Milk Acid Base Or Neutral
When it comes to the chemical nature of milk, it is essential to understand its pH level, which determines whether it is an acid, a base, or neutral. Milk is a complex biological fluid that plays a significant role in human nutrition and has unique chemical properties. In this article, we will delve into the fascinating world of milk's pH, exploring its composition, the factors that influence its acidity or alkalinity, and the practical implications of its pH status.
The pH Scale and Milk’s Position
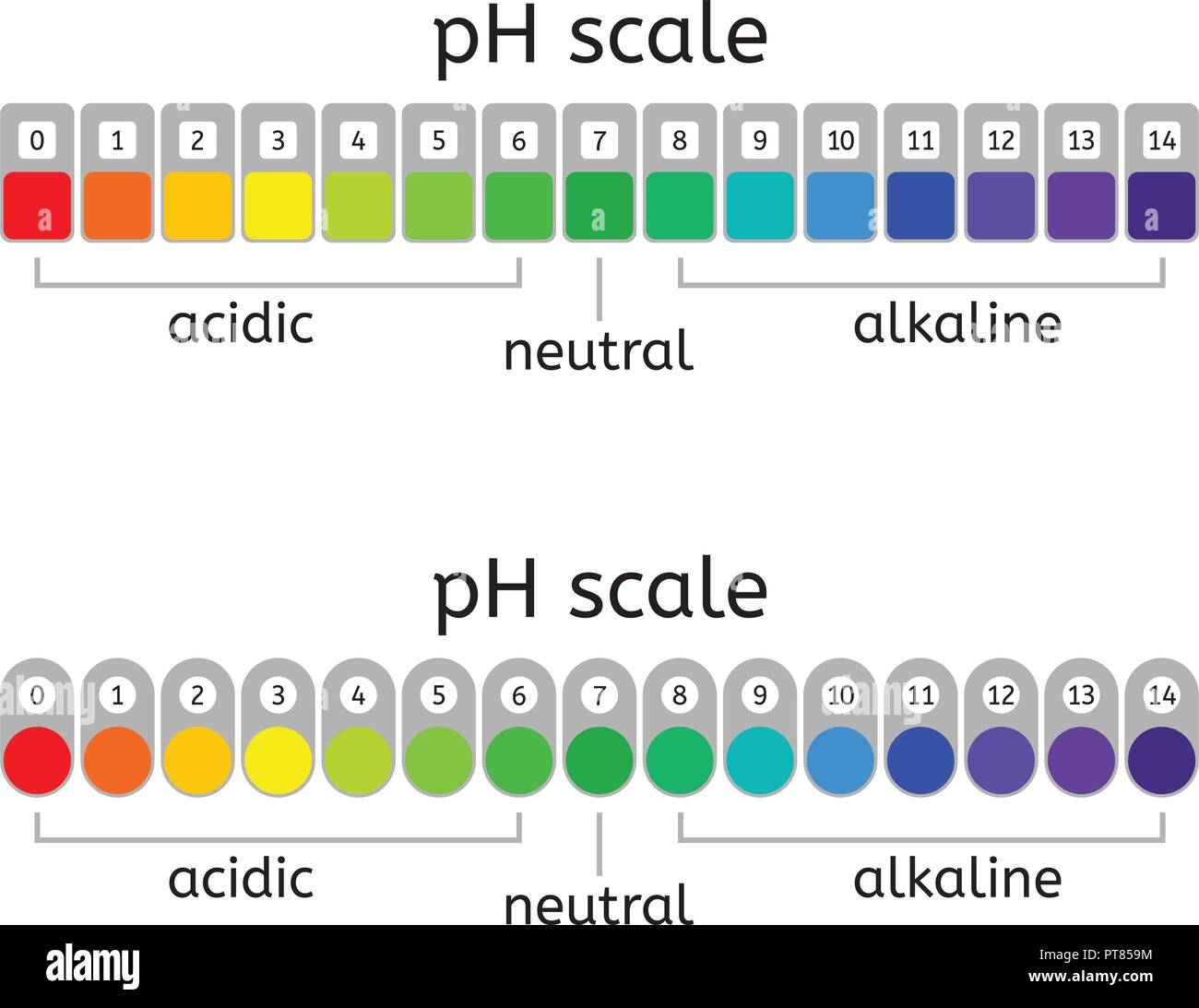
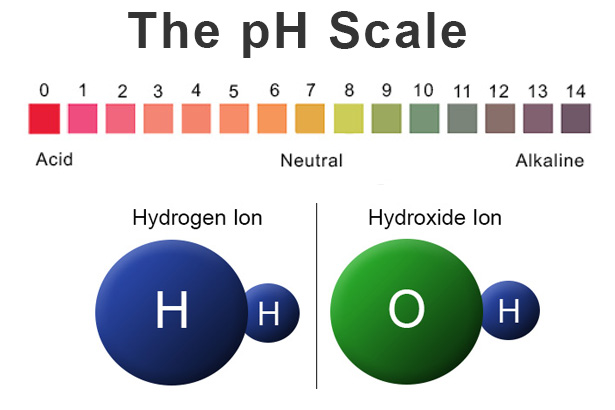
The pH scale is a crucial tool used to measure the acidity or alkalinity of a substance. It ranges from 0 to 14, with 7 being considered neutral. Any pH value below 7 indicates acidity, while values above 7 represent alkalinity or basicity. Milk, being a biological fluid, has a unique pH level that can vary depending on various factors.
Pure milk, in its natural state, typically falls within the slightly acidic to neutral range. The pH of milk can vary between 6.4 and 6.8, depending on the source, processing methods, and other environmental factors. This slight acidity is primarily due to the presence of organic acids, such as lactic acid, which is a natural byproduct of the fermentation process that occurs during milk production.
Factors Influencing Milk’s pH
Several factors can influence the pH of milk, leading to variations in its acidity or alkalinity.
- Source of Milk: The pH of milk can differ based on the animal species it originates from. For instance, cow's milk generally has a pH closer to 6.6, while goat's milk tends to be slightly more acidic, with a pH around 6.4.
- Processing and Handling: The way milk is processed and handled can impact its pH. Pasteurization, for example, can slightly increase the pH of milk due to the heat treatment involved. Additionally, storage conditions and the presence of bacteria can also affect the pH over time.
- Additives and Treatments: Certain additives or treatments applied to milk can alter its pH. For instance, the addition of sodium bicarbonate (baking soda) to milk can make it more alkaline, while the introduction of certain enzymes or bacteria can increase its acidity.
To illustrate the impact of these factors, consider the following table, which provides a comparison of the pH levels of different milk types and treatments:
| Milk Type | pH Level |
|---|---|
| Fresh Cow's Milk | 6.5-6.7 |
| Goat's Milk | 6.3-6.5 |
| Pasteurized Milk | 6.6-6.8 |
| Milk with Added Baking Soda | 7.2-7.5 |
| Fermented Milk (e.g., Yogurt) | 4.0-4.5 |
Practical Implications of Milk’s pH
The pH of milk has practical implications in various industries and everyday life. Here are some key areas where milk’s pH plays a significant role:
Nutrition and Health
Milk’s pH is closely linked to its nutritional composition and digestive properties. The slightly acidic nature of milk can enhance the absorption of certain nutrients, such as calcium and phosphorus. Additionally, the pH of milk can impact its interaction with digestive enzymes, influencing the overall digestibility of the milk proteins.
For individuals with digestive issues or certain health conditions, the pH of milk can be a crucial factor. For instance, individuals with acid reflux or gastroesophageal reflux disease (GERD) may find that the slightly acidic nature of milk can aggravate their symptoms. In such cases, choosing more alkaline milk alternatives or treating milk to increase its pH can be beneficial.
Dairy Processing and Manufacturing
The pH of milk is a critical parameter in the dairy industry, as it influences various processes and product qualities. For example, in cheese production, the pH of milk affects the curd formation, texture, and flavor of the final product. Lower pH levels can result in firmer curds and a sharper taste, while higher pH levels may produce softer curds and a milder flavor.
Additionally, the pH of milk is an important consideration in fermentation processes, such as yogurt production. The initial pH of milk can impact the growth and activity of beneficial bacteria, which in turn affects the final texture, taste, and nutritional value of the fermented product.
Milk’s Role in Baking and Cooking
In culinary applications, the pH of milk can influence the outcome of various recipes. For instance, in baking, the pH of milk can affect the rise of dough and the texture of baked goods. Lower pH levels can result in a more acidic environment, which can impact gluten development and the overall texture of the final product.
Furthermore, the pH of milk can interact with other ingredients, such as leavening agents (e.g., baking soda and baking powder). Understanding the pH of milk is crucial when adjusting recipes to achieve the desired texture, flavor, and leavening properties.
Environmental and Ecological Considerations
The pH of milk also has ecological implications, particularly in wastewater management. Milk and dairy wastewater, if not properly treated, can have a significant impact on the pH of receiving water bodies. High levels of organic acids in milk can lead to increased acidity in wastewater, potentially causing environmental damage and disrupting aquatic ecosystems.
Therefore, proper treatment and management of dairy wastewater are essential to maintain the ecological balance and protect aquatic environments.
Conclusion: Milk’s Complex Nature
Milk’s pH is a fascinating aspect of its chemical composition, offering insights into its nutritional value, industrial applications, and environmental impact. While milk is generally considered mildly acidic to neutral, its pH can vary based on numerous factors, making it a dynamic and versatile substance.
From nutritional benefits to culinary applications and environmental considerations, the pH of milk plays a pivotal role in various aspects of our daily lives. Understanding milk's pH empowers us to make informed choices, optimize its use in different industries, and appreciate the complexity of this essential biological fluid.
Can the pH of milk be altered for specific purposes?
+Yes, the pH of milk can be intentionally altered through various treatments. For instance, adding alkaline substances like sodium bicarbonate can increase the pH, making the milk more alkaline. This is often done to neutralize the acidity of milk for specific culinary applications or to enhance its digestibility for individuals with certain health conditions.
How does the pH of milk impact its nutritional value?
+The pH of milk can influence the absorption of certain nutrients. For example, the slightly acidic nature of milk enhances the absorption of calcium and phosphorus, making it an excellent source of these essential minerals. Additionally, the pH can impact the digestibility of milk proteins, which can be crucial for individuals with digestive issues.
Can the pH of milk be used to determine its freshness or quality?
+The pH of milk can provide some insights into its freshness and quality. As milk spoils or undergoes fermentation, its pH tends to decrease, becoming more acidic. However, it is important to note that pH alone is not a definitive indicator of freshness, as other factors, such as microbial activity and spoilage, can also impact milk’s quality.



