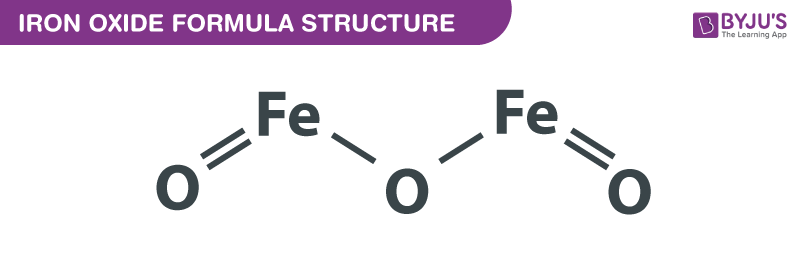
Iron 2 Oxide Formula
Iron oxide is a versatile compound with numerous applications in various industries. Its unique properties make it an essential material for everything from pigments and coatings to advanced technological processes. In this comprehensive article, we delve into the intricacies of iron oxide, exploring its chemical formula, different types, properties, uses, and the environmental impact of its production and application. We will also discuss some fascinating real-world examples of iron oxide's role in modern life.
Understanding Iron Oxide
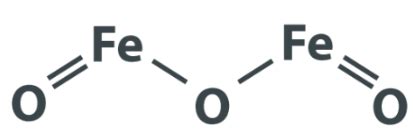
Iron oxide, often referred to as rust, is a chemical compound that occurs naturally and is composed of iron and oxygen. Its chemical formula varies depending on the specific type of iron oxide. The most common forms of iron oxide are:
- Fe2O3 - Iron(III) oxide, also known as hematite or red iron oxide.
- Fe3O4 - Iron(II,III) oxide, a mixed-valence compound commonly called magnetite.
- FeO - Iron(II) oxide, or wüstite, which is less common and unstable in standard conditions.
Each of these compounds has its unique characteristics and plays distinct roles in various industries.
Chemical Properties
Iron(III) oxide (Fe2O3) is a reddish-brown solid with a molecular weight of approximately 159.69 g/mol. It is relatively insoluble in water but can be dissolved in strong acids, releasing iron(III) cations. Hematite is the most stable form of iron oxide under standard conditions and is often used as a reference point when discussing iron oxide chemistry.
On the other hand, magnetite (Fe3O4) is a black, magnetic solid with a molecular weight of about 231.54 g/mol. It is slightly more soluble in water than hematite and has unique magnetic properties, making it valuable in various applications.
| Iron Oxide Type | Chemical Formula | Color | Solubility in Water |
|---|---|---|---|
| Hematite (Fe2O3) | Fe2O3 | Reddish-brown | Insoluble |
| Magnetite (Fe3O4) | Fe3O4 | Black | Slightly soluble |
| Wüstite (FeO) | FeO | Dark brown | Insoluble |

Physical Properties
Iron oxides exhibit various physical properties that make them valuable in different applications. Hematite, for instance, is a hard and dense mineral with a Mohs hardness of 5.5-6.5. It has a high melting point, typically around 1565-1570 °C, making it suitable for high-temperature applications.
Magnetite, due to its magnetic properties, is a key component in various electronic devices and magnetic storage media. Its magnetic susceptibility allows it to be used in magnetic resonance imaging (MRI) and other medical applications.
Production and Applications
Iron oxide is produced through a variety of processes, with the specific method depending on the desired type and purity. The most common methods include:
- Mining and Processing: Natural iron oxide deposits, such as hematite and magnetite, are mined and processed to extract the pure iron oxide.
- Synthetic Production: Iron oxides can be synthesized through chemical reactions, such as the thermal decomposition of iron(III) nitrate or the reaction between iron(II) sulfate and hydrogen peroxide.
- Recycling: Some iron oxides, particularly magnetite, can be recycled from industrial waste or by-products, reducing environmental impact.
Applications in Industry
Iron oxide finds applications across a wide range of industries due to its unique properties:
- Pigments and Coatings: Hematite and other iron oxides are widely used as pigments in paints, inks, and cosmetics. They provide a range of colors, from deep reds to earthy browns, and can also enhance the durability of coatings.
- Steel Manufacturing: Iron oxides are essential in the production of steel. They are used as feedstock in the blast furnace process, where iron ore is reduced to iron metal. This iron is then further processed to produce steel.
- Electronics and Technology: Magnetite's magnetic properties make it crucial in the production of magnetic storage media, such as hard drives and magnetic tapes. It is also used in various electronic components and sensors.
- Environmental Remediation: Certain iron oxides, like magnetite, can be used for water treatment and environmental remediation. They can remove contaminants from water and soil, making them valuable in sustainable practices.
- Medicine: Iron oxides have found applications in medical imaging and drug delivery systems. For instance, superparamagnetic iron oxide nanoparticles (SPIONs) are used in MRI contrast agents and targeted drug delivery.
Real-World Examples
Iron oxide’s impact on our daily lives is extensive. Here are a few real-world examples:
- The vibrant red hues in many famous paintings, including those by the Impressionists, often come from natural iron oxide pigments.
- The steel used in the construction of skyscrapers and bridges relies on iron oxide as a key component in its production.
- Hard drives and memory storage devices in our computers and smartphones utilize magnetite's magnetic properties.
- Iron oxide nanoparticles are being explored for targeted cancer treatment, offering a promising avenue for personalized medicine.
Environmental Considerations
While iron oxide is an essential material, its production and use can have environmental implications. Mining and processing iron oxide can lead to habitat destruction and water pollution if not managed sustainably. However, recycling and responsible mining practices can mitigate these impacts.
Additionally, the use of iron oxide nanoparticles, while promising in medical applications, raises concerns about their potential environmental impact. Research is ongoing to understand and manage these risks effectively.
Frequently Asked Questions
How is iron oxide formed naturally in the environment?
+Iron oxide forms naturally through the oxidation of iron-containing minerals, particularly under conditions of high humidity and temperature. This process, known as rusting, is a common occurrence in environments with high iron content, such as soil or water bodies.
What are the main differences between hematite and magnetite?
+Hematite (Fe2O3) and magnetite (Fe3O4) are both iron oxides, but they differ in their chemical composition and properties. Hematite is a reddish-brown solid with a higher iron content and is more stable under standard conditions. Magnetite is a black, magnetic solid with a lower iron content and unique magnetic properties.
Can iron oxide be recycled, and what are the benefits?
+Yes, iron oxide, particularly magnetite, can be recycled from industrial waste or by-products. Recycling iron oxide reduces the need for mining new iron ore, conserves resources, and minimizes environmental impact. It also contributes to a more sustainable and circular economy.
What are the potential risks of using iron oxide nanoparticles in medicine?
+Iron oxide nanoparticles, while offering promising medical applications, also present potential risks. These include toxicity to cells and tissues, as well as environmental concerns if not properly managed. Research is ongoing to ensure their safe and effective use in medical treatments.