Understanding Hydrogen Cyanide: 3 Key Facts
Hydrogen cyanide, often denoted as HCN, is a highly toxic chemical compound that has garnered significant attention due to its unique properties and diverse applications. This colorless gas with a faint, bitter almond-like odor has a rich history and a wide range of industrial uses, despite its deadly nature. In this article, we delve into the world of hydrogen cyanide, uncovering three key facts that shed light on its importance, risks, and intriguing characteristics.
The Chemical Composition and Reactivity of Hydrogen Cyanide

Hydrogen cyanide is a simple molecule consisting of one hydrogen atom, one carbon atom, and one nitrogen atom. Its chemical formula, HCN, reflects this straightforward composition. However, don’t let its simplicity deceive you; hydrogen cyanide is an incredibly reactive and versatile compound. One of its remarkable properties is its ability to form strong hydrogen bonds, which contribute to its unique behavior in various chemical reactions.
In the presence of water, hydrogen cyanide undergoes a process known as hydrolysis. This reaction leads to the formation of hydrogen ions (H+) and cyanide ions (CN−). The cyanide ion is highly toxic and can rapidly interfere with cellular processes, particularly by inhibiting an essential enzyme called cytochrome c oxidase. This inhibition disrupts cellular respiration, leading to a rapid and often fatal outcome.
Furthermore, hydrogen cyanide's reactivity extends beyond its interaction with water. It readily combines with various compounds, forming complex molecules. This property makes it a valuable reagent in organic synthesis, where it is used to create a wide array of organic compounds, including pharmaceuticals, dyes, and agricultural chemicals.
Hydrogen Cyanide in Organic Synthesis: A Double-Edged Sword
The utilization of hydrogen cyanide in organic synthesis is a prime example of its dual nature. On one hand, it serves as a crucial building block for the creation of numerous valuable compounds. Its ability to form stable adducts with metals and its participation in various chemical reactions make it an indispensable tool in the chemist’s toolkit.
However, the same reactivity that makes hydrogen cyanide so useful also poses significant challenges. Its toxicity demands careful handling and strict safety protocols in laboratory and industrial settings. Accidental exposure or improper handling can lead to severe health consequences, including respiratory distress, neurological damage, and even death.
Researchers and industries have developed rigorous safety measures to mitigate these risks. These include the use of specialized equipment, such as fume hoods and personal protective gear, along with comprehensive training programs to ensure the safe handling and storage of hydrogen cyanide.
| Safety Precautions | Key Measures |
|---|---|
| Ventilation | Adequate ventilation systems to prevent buildup |
| PPE | Respiratory protection, gloves, and eye protection |
| Training | Comprehensive safety training for all personnel |
| Storage | Secure, temperature-controlled storage areas |

The Historical Significance and Applications of Hydrogen Cyanide
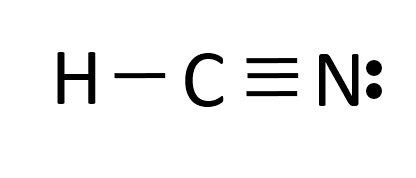
Hydrogen cyanide’s impact extends beyond its chemical properties; it has played a significant role in human history and continues to find diverse applications in various industries.
A Historical Perspective: From Deadly Gas to Synthetic Fiber
During World War I, hydrogen cyanide gained notoriety as a deadly chemical warfare agent. Its ability to rapidly incapacitate and kill made it a feared weapon on the battlefield. However, the post-war period saw a shift in its perception and utilization.
In the 1930s, scientists discovered that hydrogen cyanide could be used as a precursor in the production of synthetic fibers, particularly polyacrylonitrile (PAN). This breakthrough led to the development of the first commercially successful synthetic fiber, nylon, which revolutionized the textile industry. Today, PAN fibers are widely used in the production of clothing, carpets, and industrial applications.
The historical evolution of hydrogen cyanide's role highlights the importance of understanding a substance's potential risks and benefits. From a lethal weapon to a key component in the textile industry, hydrogen cyanide's journey is a testament to the intricate relationship between science, technology, and society.
Modern Applications: Beyond Textiles
While its use in synthetic fibers remains significant, hydrogen cyanide’s applications have expanded into other domains. In the pharmaceutical industry, it is employed as a precursor for the synthesis of various drugs, including antidepressants and painkillers. Its reactivity allows for the creation of complex molecular structures essential for these medications.
Additionally, hydrogen cyanide finds use in chemical analysis and detection methods. Its unique chemical properties make it a valuable tool in analytical chemistry, where it is used to identify and quantify various compounds. In environmental monitoring, hydrogen cyanide-based assays are employed to detect and measure cyanide levels in water and soil samples, ensuring the safety of ecosystems.
| Industry | Hydrogen Cyanide Applications |
|---|---|
| Textiles | Production of synthetic fibers like nylon and PAN |
| Pharmaceuticals | Synthesis of drugs, including antidepressants and painkillers |
| Chemical Analysis | Assays for cyanide detection in environmental samples |
The Future of Hydrogen Cyanide: Safety, Sustainability, and Innovation
As we move forward, the focus on hydrogen cyanide’s future revolves around three key aspects: safety, sustainability, and innovation.
Enhancing Safety Measures
The inherent toxicity of hydrogen cyanide underscores the need for continuous improvement in safety protocols. Researchers and industries are dedicated to developing advanced safety systems and training programs to minimize the risks associated with its handling and storage. Additionally, efforts are being made to explore alternative methods for its synthesis and utilization, reducing the potential for accidental exposure.
Sustainable Practices
In an era of growing environmental consciousness, the sustainable production and use of hydrogen cyanide are gaining prominence. Researchers are exploring ways to reduce the environmental impact of its synthesis and disposal. This includes the development of green synthesis methods that minimize the use of hazardous reagents and by-products, as well as the implementation of recycling and recovery processes to maximize resource efficiency.
Innovation in Applications
The unique properties of hydrogen cyanide continue to inspire innovation. Scientists are investigating its potential in emerging fields, such as nanotechnology and energy storage. In nanotechnology, hydrogen cyanide’s ability to form stable adducts with metal ions opens up possibilities for the creation of novel materials with enhanced properties. In energy storage, its reactivity and high energy density make it a promising candidate for next-generation batteries and fuel cells.
The future of hydrogen cyanide is promising, with ongoing research and development efforts shaping its role in a wide range of industries. As we continue to unlock its potential, a balanced approach that prioritizes safety, sustainability, and innovation will be crucial to harnessing its benefits while mitigating its risks.
How is hydrogen cyanide produced commercially?
+Commercial production of hydrogen cyanide typically involves the Andrussow process, where methane and ammonia are reacted at high temperatures in the presence of oxygen and a platinum catalyst. This process yields hydrogen cyanide with high purity.
What are the symptoms of hydrogen cyanide exposure?
+Symptoms of hydrogen cyanide exposure can include rapid breathing, dizziness, headache, nausea, and confusion. In severe cases, it can lead to loss of consciousness, respiratory failure, and death.
How is hydrogen cyanide used in agriculture?
+Hydrogen cyanide is used in agriculture as a fumigant to control pests and diseases in stored grains and seeds. It is also used in the production of certain agricultural chemicals and fertilizers.



