A Simple Guide to HCL Lewis Structures
Welcome to a comprehensive guide on understanding and drawing HCL Lewis structures, a fundamental concept in chemistry that provides a visual representation of the bonding and electron distribution within molecules. Lewis structures, also known as electron dot structures, are essential tools for chemists and students alike, offering insights into the nature of chemical bonds and molecular geometry. In this guide, we will delve into the intricacies of HCL Lewis structures, providing a step-by-step approach to their creation and interpretation.
The Basics of Lewis Structures
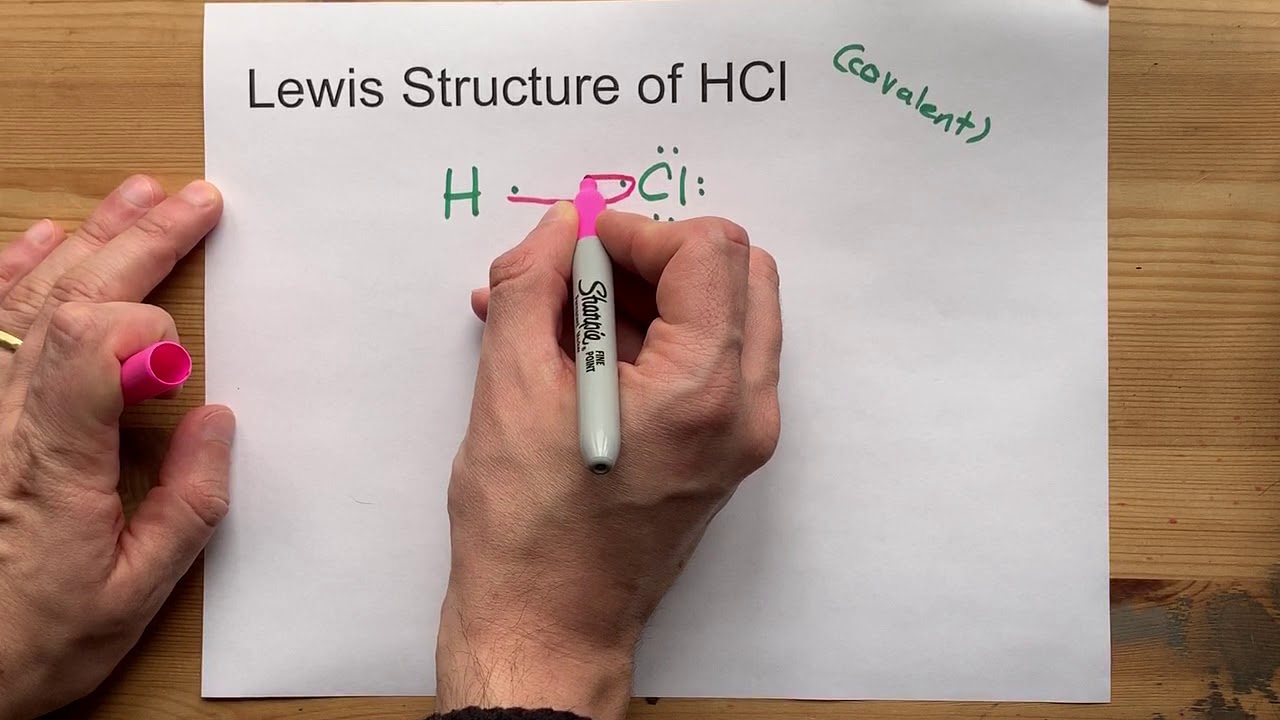
Lewis structures, named after the American chemist Gilbert N. Lewis, serve as a means to depict the distribution of electrons in molecules and ions. They are particularly useful in illustrating covalent bonds, where electrons are shared between atoms, and lone pairs, where electrons are not involved in bonding. These structures provide a simplified yet powerful representation of molecular bonding, making them an indispensable tool in the field of chemistry.
Understanding Electron Configuration
Before we dive into the specifics of HCL Lewis structures, it’s crucial to understand the electron configuration of atoms. Each element in the periodic table has a unique electron configuration, which determines its chemical behavior and bonding preferences. Electrons in atoms occupy different energy levels or shells, with each shell accommodating a specific number of electrons. The outermost shell, known as the valence shell, is of particular interest in Lewis structures as it contains the electrons involved in bonding.
For instance, consider the element Hydrogen (H), which has one electron in its valence shell, and Chlorine (Cl), which has seven electrons in its valence shell. These valence electrons play a critical role in the formation of chemical bonds.
Constructing HCL Lewis Structures
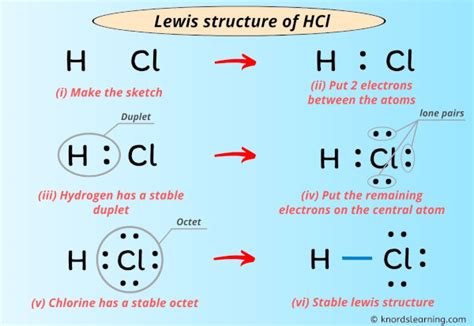
Now, let’s focus on constructing the Lewis structure for the molecule Hydrogen Chloride (HCL). This binary molecular compound is formed by the bonding of a single hydrogen atom and a single chlorine atom. Here’s a step-by-step guide to creating its Lewis structure:
Step 1: Count Valence Electrons
Begin by determining the total number of valence electrons in the molecule. For HCL, Hydrogen has one valence electron, and Chlorine has seven valence electrons. Therefore, the total number of valence electrons in the HCL molecule is 8.
Step 2: Draw the Molecular Skeleton
Next, draw the skeletal structure of the molecule, placing the atoms according to their connectivity. In the case of HCL, Hydrogen is bonded to Chlorine, so draw them as such:
| H | — | Cl |
Step 3: Draw Bonding Electrons
Now, let’s distribute the valence electrons to form bonds. Start by pairing up the electrons between the atoms. In HCL, one electron from Hydrogen pairs with one electron from Chlorine to form a single covalent bond:
| H | — | Cl |
| ⋅ | ≡ | ⋅ |
Step 4: Add Lone Pair Electrons
With the bonding electrons accounted for, we now need to add the remaining valence electrons as lone pairs. Hydrogen has no remaining electrons, so it doesn’t need any lone pairs. Chlorine, on the other hand, has six remaining electrons, so we add them as three lone pairs:
| H | — | Cl |
| ⋅ | ≡ | ⋅ |
| ⋅⋅⋅ | ||
| ⋅⋅⋅ |
Step 5: Check for Stability
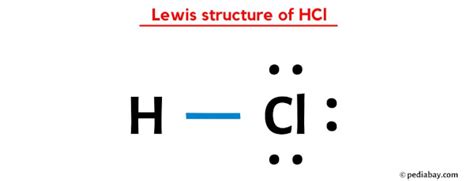
The Lewis structure of HCL is now complete. However, it’s important to ensure that the structure is stable. In this case, both Hydrogen and Chlorine have a full valence shell, making the molecule stable. The Hydrogen atom has achieved a stable electron configuration similar to that of the noble gas Helium (He), while the Chlorine atom has achieved a stable configuration similar to Argon (Ar). This stability is a key feature of Lewis structures, as it indicates the molecule’s tendency to form or break bonds.
Interpreting HCL Lewis Structure
The Lewis structure of HCL provides valuable insights into the molecule’s bonding and electron distribution. Here’s a breakdown of what we can interpret from this structure:
Bonding in HCL
The single covalent bond between Hydrogen and Chlorine indicates a sharing of one electron pair between the two atoms. This bond is formed due to the need of both atoms to complete their valence shells. Hydrogen, with its one electron, finds stability by sharing it with Chlorine, while Chlorine, with its seven electrons, needs one more to complete its octet.
Electron Distribution
The Lewis structure also shows that Chlorine has three lone pairs of electrons, indicating that it has a negative charge. This negative charge is balanced by the positive charge of Hydrogen, resulting in a neutral overall charge for the molecule. This distribution of charges is crucial in understanding the molecule’s reactivity and its interactions with other species.
Molecular Geometry
While Lewis structures primarily focus on bonding and electron distribution, they can also provide hints about molecular geometry. In the case of HCL, the linear structure with the bond and lone pairs on opposite sides suggests a linear molecular geometry. This geometry is consistent with the molecule’s simple bonding pattern and the need for maximum separation of electron pairs to minimize repulsive forces.
Practical Applications and Considerations
Understanding Lewis structures is not just a theoretical exercise; it has practical applications in various fields of chemistry. Here are some key considerations and applications of Lewis structures:
Chemical Bonding and Reactions
Lewis structures are invaluable in predicting and explaining chemical reactions. By analyzing the electron distribution and bonding patterns, chemists can anticipate the formation or breaking of bonds, leading to the understanding of reaction mechanisms and the design of new compounds.
Molecular Polarity
Lewis structures help determine the polarity of molecules. In HCL, the unequal distribution of electrons between Hydrogen and Chlorine results in a polar bond, with a partial positive charge on Hydrogen and a partial negative charge on Chlorine. This polarity affects the molecule’s solubility and reactivity in different environments.
Resonance Structures
In some cases, a molecule may have multiple possible Lewis structures, known as resonance structures. These structures represent different electron distributions that contribute to the overall stability of the molecule. For example, Ozone (O3) has multiple resonance structures due to the delocalization of its electrons.
Octet Rule and Exceptions
While the octet rule, which states that atoms tend to have eight electrons in their valence shell, is a useful guideline, it has exceptions. Some atoms, like Boron (B) and Aluminum (Al), are stable with less than eight valence electrons, while others, like Sulfur (S) and Phosphorus (P), can accommodate more than eight electrons. Lewis structures help identify and understand these exceptions.
Limitations of Lewis Structures
While Lewis structures are powerful tools, they have limitations. They are simplified representations and may not capture the dynamic nature of electron distribution in more complex molecules. Additionally, Lewis structures do not provide information about molecular orbitals or the precise spatial arrangement of electrons, which can be crucial in understanding certain molecular properties.
Conclusion

In this comprehensive guide, we’ve explored the fundamentals of HCL Lewis structures, from their construction to their interpretation. We’ve delved into the intricacies of electron configuration, bonding, and stability, and discussed the practical applications and limitations of Lewis structures. By mastering the art of drawing and interpreting Lewis structures, you’ll gain a deeper understanding of chemical bonding and the fascinating world of molecular chemistry.
How do Lewis structures help in predicting molecular geometry?
+Lewis structures provide a visual representation of electron distribution and bonding, which directly influences molecular geometry. By analyzing the arrangement of bonding and lone pair electrons, you can predict the shape and spatial arrangement of the molecule, aiding in the understanding of its physical and chemical properties.
What are some common exceptions to the octet rule?
+The octet rule states that atoms tend to have eight electrons in their valence shell, but there are exceptions. Elements like Boron (B) and Aluminum (Al) are stable with less than eight valence electrons, while elements like Sulfur (S) and Phosphorus (P) can accommodate more than eight electrons. Lewis structures help identify and understand these exceptions.
How do resonance structures contribute to molecular stability?
+Resonance structures represent multiple possible electron distributions for a molecule, where each structure has a certain degree of stability. These structures contribute to the overall stability of the molecule by allowing for a more evenly distributed electron density, reducing the strain on individual bonds.