Formula Sodium Fluoride
Sodium fluoride (NaF) is a chemical compound that has found significant applications in various industries, particularly in dentistry and water treatment. Its unique properties make it an essential component in preventing dental caries and controlling water quality. This article delves into the chemistry, production, applications, and safety considerations associated with sodium fluoride.
Chemical Properties and Structure

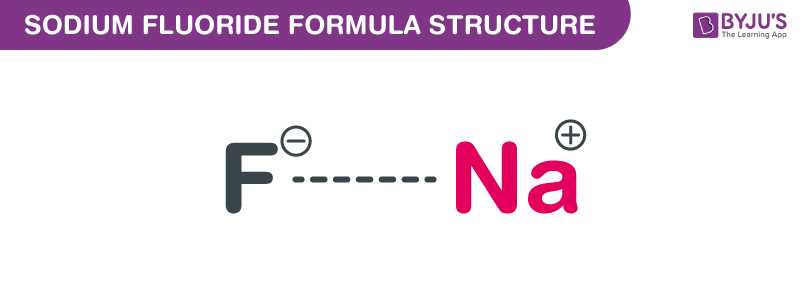
Sodium fluoride is an ionic compound, composed of sodium cations (Na+) and fluoride anions (F-). It has a cubic crystal structure, forming a white, odorless solid at room temperature. The chemical formula, NaF, represents the 1:1 ratio of sodium to fluoride ions in the compound.
The ionic bond between sodium and fluoride ions results in a highly stable compound. This stability is crucial for its applications, as it ensures the compound's integrity and effectiveness over time. The melting point of sodium fluoride is approximately 993°C, and its boiling point is significantly higher, around 1700°C.
Production and Synthesis

Sodium fluoride is primarily produced through a chemical reaction between hydrofluoric acid (HF) and sodium hydroxide (NaOH). This process, known as neutralization, yields sodium fluoride as a byproduct. The equation for this reaction is as follows:
HF + NaOH → NaF + H2O
Hydrofluoric acid, derived from fluorspar (fluorite, CaF2), is a key raw material in the production of sodium fluoride. The reaction between HF and NaOH is exothermic, releasing heat and forming a soluble salt, NaF, along with water.
Alternatively, sodium fluoride can be synthesized by treating sodium carbonate (Na2CO3) with hydrofluoric acid. This reaction yields sodium fluoride and carbon dioxide (CO2). The equation for this synthesis is:
2HF + Na2CO3 → 2NaF + H2O + CO2
The choice of production method depends on the availability and cost of raw materials, as well as the desired purity of the final product.
Applications of Sodium Fluoride
Dental Care and Oral Health
One of the most well-known applications of sodium fluoride is in dentistry. It is widely used in toothpaste, mouthwashes, and dental treatments to prevent tooth decay and promote oral health.
When sodium fluoride is applied to the teeth, it interacts with the enamel, forming a protective layer of fluorapatite. This layer is more resistant to acid attacks from plaque bacteria, thereby reducing the risk of cavities. The fluoride ion also has the ability to remineralize early dental lesions, reversing the initial stages of tooth decay.
In addition to its direct application in oral care products, sodium fluoride is also used in community water fluoridation programs. By adjusting the fluoride levels in drinking water to optimal concentrations, public health authorities can significantly reduce dental caries prevalence among the population.
Water Treatment and Quality Control
Sodium fluoride finds extensive use in water treatment processes. It is added to water supplies to control the growth of bacteria and algae, preventing the formation of biofilms and maintaining water clarity.
Fluoride also plays a crucial role in corrosion inhibition. When added to water, it forms a protective film on the inner surfaces of pipes and storage tanks, preventing corrosion and ensuring the longevity of the water infrastructure.
Furthermore, sodium fluoride is utilized in the treatment of industrial wastewater. Its ability to precipitate heavy metals, such as lead and mercury, makes it an effective agent for removing these contaminants from wastewater streams, thus ensuring environmental safety.
Other Industrial Applications
Beyond its dental and water treatment applications, sodium fluoride has a range of industrial uses:
- Pharmaceuticals: It is used as an excipient in tablet formulations and as an active ingredient in certain medications.
- Pest Control: Sodium fluoride is employed as an insecticide and rodenticide, targeting pests such as cockroaches and rats.
- Analytical Chemistry: In laboratory settings, sodium fluoride is used as a matrix material in inductively coupled plasma mass spectrometry (ICP-MS) to enhance the detection of trace elements.
Safety Considerations
While sodium fluoride has numerous beneficial applications, it is essential to handle and use it with caution due to its toxic nature. Ingestion of excessive amounts can lead to fluorosis, a condition characterized by tooth and bone damage.
To ensure safe handling, strict adherence to safety guidelines is necessary. Personal protective equipment, including gloves and eye protection, should be worn when working with sodium fluoride. Additionally, proper ventilation and spill response protocols must be in place to mitigate potential risks.
The use of sodium fluoride in dental products and water treatment is carefully regulated to maintain safe concentrations. In dental care, fluoride levels are controlled to prevent over-fluoridation and potential harm to oral tissues. Similarly, water fluoridation programs are meticulously monitored to ensure optimal fluoride levels for dental health without causing adverse effects.
| Property | Value |
|---|---|
| Chemical Formula | NaF |
| Melting Point | 993°C |
| Boiling Point | 1700°C |
| Density | 2.556 g/cm3 |
| Solubility in Water | Soluble |

How does sodium fluoride prevent tooth decay?
+Sodium fluoride interacts with tooth enamel, forming a protective layer of fluorapatite. This layer is more resistant to acid attacks from plaque bacteria, reducing the risk of cavities. Additionally, fluoride can remineralize early dental lesions, reversing the initial stages of tooth decay.
What are the safety precautions when handling sodium fluoride?
+Due to its toxic nature, strict safety guidelines must be followed when handling sodium fluoride. This includes wearing personal protective equipment, ensuring proper ventilation, and having spill response protocols in place. Ingestion of excessive amounts can lead to fluorosis, so caution is necessary.
How is sodium fluoride used in water treatment?
+Sodium fluoride is added to water supplies to control the growth of bacteria and algae, maintaining water clarity. It also acts as a corrosion inhibitor, forming a protective film on the inner surfaces of pipes and tanks. Additionally, it is used to precipitate heavy metals from industrial wastewater, ensuring environmental safety.


