Flow Chart Classification Of Matter
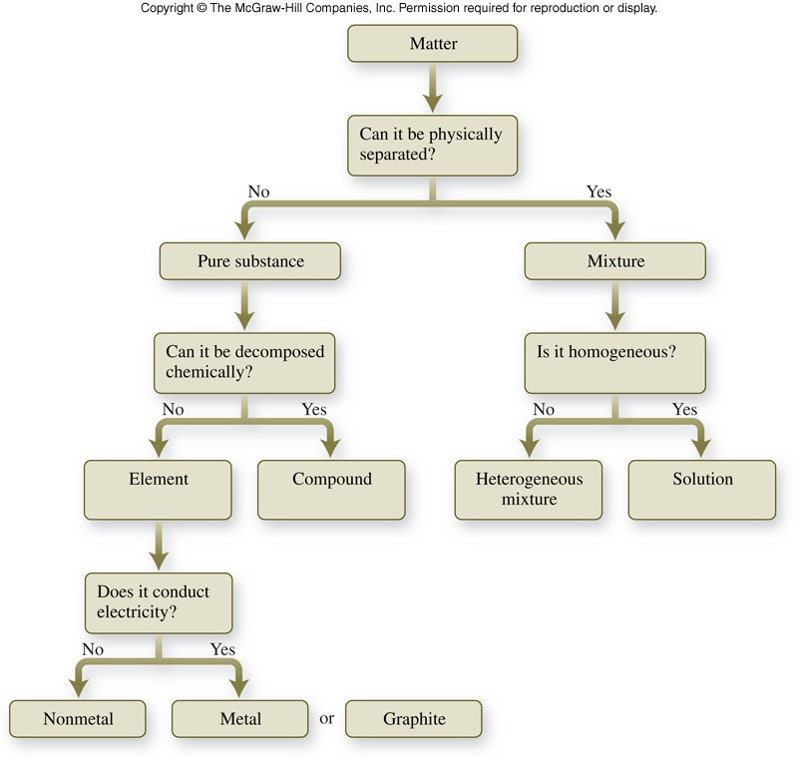
Welcome to an in-depth exploration of the fascinating world of matter classification, a fundamental concept in the realm of chemistry. Understanding how substances are categorized is crucial for chemists, as it forms the basis for further exploration and experimentation. In this comprehensive guide, we will delve into the intricacies of the flow chart classification of matter, a systematic approach that helps chemists and scientists organize and understand the diverse world of substances. By the end of this article, you'll have a solid grasp of this essential classification system and its significance in the field of chemistry.
The Fundamentals of Matter Classification

Matter, in its simplest definition, is anything that occupies space and has mass. It is the stuff that makes up our universe, from the air we breathe to the stars in the sky. The classification of matter is a systematic way to organize and categorize these substances based on their physical and chemical properties. This classification is essential for various reasons, including ease of study, prediction of behavior, and identification of unknown substances.
The flow chart classification of matter is a hierarchical system, which means it starts with broad categories and gradually narrows down to more specific ones. This method allows for a comprehensive and logical organization of matter, making it an invaluable tool for chemists and researchers.
The First Step: Pure Substances vs Mixtures
The initial step in the flow chart classification of matter is to distinguish between pure substances and mixtures. Pure substances are those that have a uniform and definite composition throughout. They cannot be separated into other substances by physical means. In contrast, mixtures are combinations of two or more substances that can be physically separated. This initial distinction forms the basis for further classification.
For instance, consider water. It is a pure substance because it always has the same composition (two hydrogen atoms and one oxygen atom) regardless of its source. On the other hand, a mixture like saltwater can be physically separated into its components - water and salt - by methods such as evaporation.
| Pure Substance | Mixture |
|---|---|
| Water (H2O) | Saltwater |
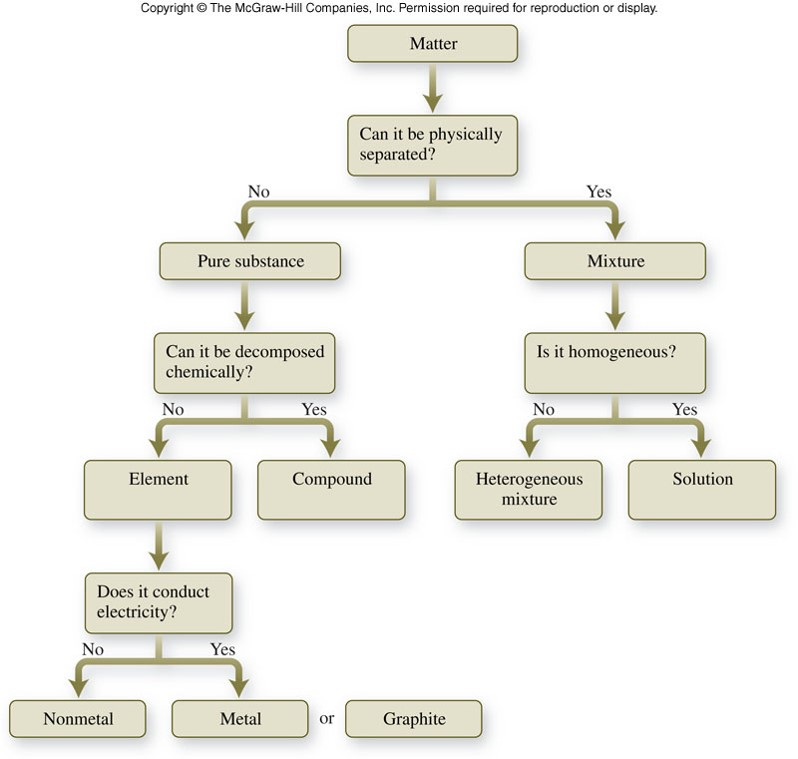
Exploring Pure Substances: Elements and Compounds
Upon identifying a pure substance, the next step is to differentiate between elements and compounds. Elements are the simplest forms of matter that cannot be broken down into simpler substances by chemical means. They are the building blocks of all matter. Compounds, on the other hand, are substances formed by the chemical combination of two or more elements in fixed proportions.
For example, hydrogen (H2) is an element, as it is the simplest form of matter and cannot be broken down further. However, water (H2O) is a compound, formed by the chemical combination of hydrogen and oxygen in a fixed 2:1 ratio.
| Element | Compound |
|---|---|
| Hydrogen (H2) | Water (H2O) |
Delving Deeper: Types of Compounds
Compounds can be further classified into two main types: molecular compounds and ionic compounds. Molecular compounds are formed when atoms of different elements share electrons to form molecules. These molecules have distinct properties and behave as individual units. Ionic compounds, on the other hand, are formed when one or more electrons are transferred from one element to another, resulting in positively charged ions (cations) and negatively charged ions (anions) that are held together by electrostatic forces.
A classic example of a molecular compound is water (H2O), where hydrogen and oxygen atoms share electrons to form a molecule. An example of an ionic compound is table salt (NaCl), where sodium donates an electron to chlorine, resulting in positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). These ions are held together by electrostatic forces to form the compound.
| Molecular Compound | Ionic Compound |
|---|---|
| Water (H2O) | Table Salt (NaCl) |
The World of Mixtures: Homogeneous and Heterogeneous

Having understood the classification of pure substances, we now turn our attention to mixtures. Mixtures are combinations of two or more substances that are not chemically combined. They can be classified into two main types: homogeneous mixtures and heterogeneous mixtures.
Homogeneous Mixtures: Solutions
Homogeneous mixtures, often referred to as solutions, are those in which the components are uniformly distributed throughout the mixture. They have a consistent composition and appear visually as a single phase. Solutions can be further classified based on the state of matter of the solute (the substance being dissolved) and the solvent (the substance doing the dissolving). Common types of solutions include solid-solid solutions, solid-liquid solutions, and gas-liquid solutions.
For instance, a solution of salt in water (sodium chloride dissolved in water) is a solid-liquid solution. Another example is air, which is a gas-liquid solution of various gases (such as oxygen, nitrogen, and carbon dioxide) dissolved in a liquid (water vapor) at atmospheric pressure.
| Solid-Liquid Solution | Gas-Liquid Solution |
|---|---|
| Saltwater (NaCl in H2O) | Air (O2, N2, CO2 in H2O) |
Heterogeneous Mixtures: Suspensions, Colloids, and Others
Heterogeneous mixtures, in contrast, are those in which the components are not uniformly distributed. They may appear as multiple phases or have visible boundaries between different parts of the mixture. Heterogeneous mixtures can include various types, such as suspensions, colloids, and emulsions.
A suspension is a heterogeneous mixture in which solid particles are dispersed in a liquid medium but do not dissolve. An example is a mixture of sand and water, where the sand particles settle over time due to their weight. A colloid, on the other hand, is a type of mixture where one substance is dispersed as very small particles (colloidal particles) throughout another substance. Milk is an example of a colloid, where fat globules are dispersed in water. An emulsion is a type of colloid where both the dispersed phase and the dispersion medium are liquids. An example is mayonnaise, where oil (as the dispersed phase) is dispersed in water (as the dispersion medium) with the help of an emulsifier.
| Suspension | Colloid | Emulsion |
|---|---|---|
| Sand in Water | Milk | Mayonnaise |
Beyond the Basics: Special Cases and Complex Mixtures
While the flow chart classification of matter provides a comprehensive framework, there are certain special cases and complex mixtures that may not fit neatly into these categories. These include alloys, interstitial compounds, and complex organic mixtures. Understanding these special cases is crucial for a more nuanced understanding of matter classification.
Alloys: Unique Mixtures of Metals
Alloys are unique mixtures of metals with often enhanced properties compared to the individual metals. They are formed by the mixing of two or more metallic elements in a solid solution, which can result in improved strength, corrosion resistance, or other desirable properties. Alloys can be classified as substitutional alloys, where one metal replaces another in a crystal lattice, or interstitial alloys, where smaller atoms fit into the spaces (interstices) between larger atoms in a crystal lattice.
A well-known example of an alloy is brass, which is a combination of copper and zinc. Brass has a higher malleability and a lower melting point than either copper or zinc alone, making it useful for a variety of applications such as in musical instruments and plumbing fixtures.
| Substitutional Alloy | Interstitial Alloy |
|---|---|
| Brass (Cu + Zn) | Steel (Fe + C) |
Interstitial Compounds: A Special Type of Compound
Interstitial compounds are a special type of compound where small atoms (usually non-metals) fit into the spaces (interstices) between larger atoms (usually metals) in a crystal lattice. These compounds have unique properties and are often used in specific applications due to their high strength, hardness, or other desirable characteristics.
One example of an interstitial compound is graphite (C), where carbon atoms fit into the interstices between layers of carbon atoms in a hexagonal lattice. Another example is interstitial carbides, such as titanium carbide (TiC), where carbon atoms fit into the interstices of a metal lattice.
| Graphite (C) | Titanium Carbide (TiC) |
|---|---|
| Carbon atoms fit into hexagonal lattice | Carbon atoms fit into metal lattice |
Organic Mixtures: A Complex World
Organic mixtures are incredibly diverse and complex, encompassing a wide range of substances derived from living organisms or produced synthetically. These mixtures can include natural products, such as oils, fats, and proteins, as well as synthetic compounds, such as plastics, pharmaceuticals, and pesticides. The classification of organic mixtures is often based on their chemical structure, functional groups, and properties.
For example, crude oil is a complex mixture of various organic compounds, including alkanes, alkenes, and aromatic compounds. It is classified based on its chemical composition and the presence of specific functional groups, such as carbon-carbon double bonds or aromatic rings.
Conclusion: The Significance of Matter Classification
The flow chart classification of matter is an invaluable tool for chemists and scientists, providing a systematic and logical way to organize and understand the diverse world of substances. This classification system allows for easier study, prediction of behavior, and identification of unknown substances. It forms the foundation for further exploration and experimentation in the field of chemistry.
From the basic distinction between pure substances and mixtures to the complex world of organic mixtures, each step in the flow chart classification offers a deeper understanding of the properties and behaviors of matter. This knowledge is crucial not only for scientific research but also for various applications in industry, medicine, and our daily lives.
In conclusion, the flow chart classification of matter is a powerful tool that enables us to make sense of the complex and fascinating world of substances. By understanding this classification, we can better appreciate the intricacies of the natural world and the ways in which we interact with it.
Frequently Asked Questions (FAQ)
What is the purpose of classifying matter?
+
Classifying matter serves several important purposes. It provides a systematic way to organize and understand the diverse world of substances, making it easier to study, predict behavior, and identify unknown substances. Classification also helps in understanding the properties and behaviors of matter, which is crucial for scientific research and various applications in industry, medicine, and daily life.
How are pure substances different from mixtures?
+
Pure substances have a uniform and definite composition throughout, and they cannot be separated into other substances by physical means. In contrast, mixtures are combinations of two or more substances that can be physically separated. This distinction forms the basis for further classification of matter.
What are the main types of compounds?
+
The main types of compounds are molecular compounds and ionic compounds. Molecular compounds are formed when atoms of different elements share electrons to form molecules, while ionic compounds are formed when one or more electrons are transferred from one element to another, resulting in positively charged ions (cations) and negatively charged ions (anions) held together by electrostatic forces.



