Draw Oxygen Difluoride: 3 Simple Steps

Oxygen Difluoride: Unlocking the Secrets of a Unique Chemical Compound
In the fascinating world of chemistry, certain compounds capture our attention with their unique properties and intriguing structures. One such compound is oxygen difluoride (OF2), a fascinating molecule that has intrigued scientists and students alike. In this article, we will delve into the world of oxygen difluoride, exploring its structure, properties, and significance, while guiding you through a simple yet engaging process to draw this molecule. So, fasten your lab coats and prepare to embark on a chemical adventure!
Understanding Oxygen Difluoride: A Quick Overview

Oxygen difluoride, often referred to as OF2, is a polar covalent molecule composed of one oxygen atom and two fluorine atoms. Its unique molecular formula and chemical behavior set it apart from many other compounds. OF2 is a highly reactive and unstable substance, which adds to its allure and makes it an intriguing subject of study.
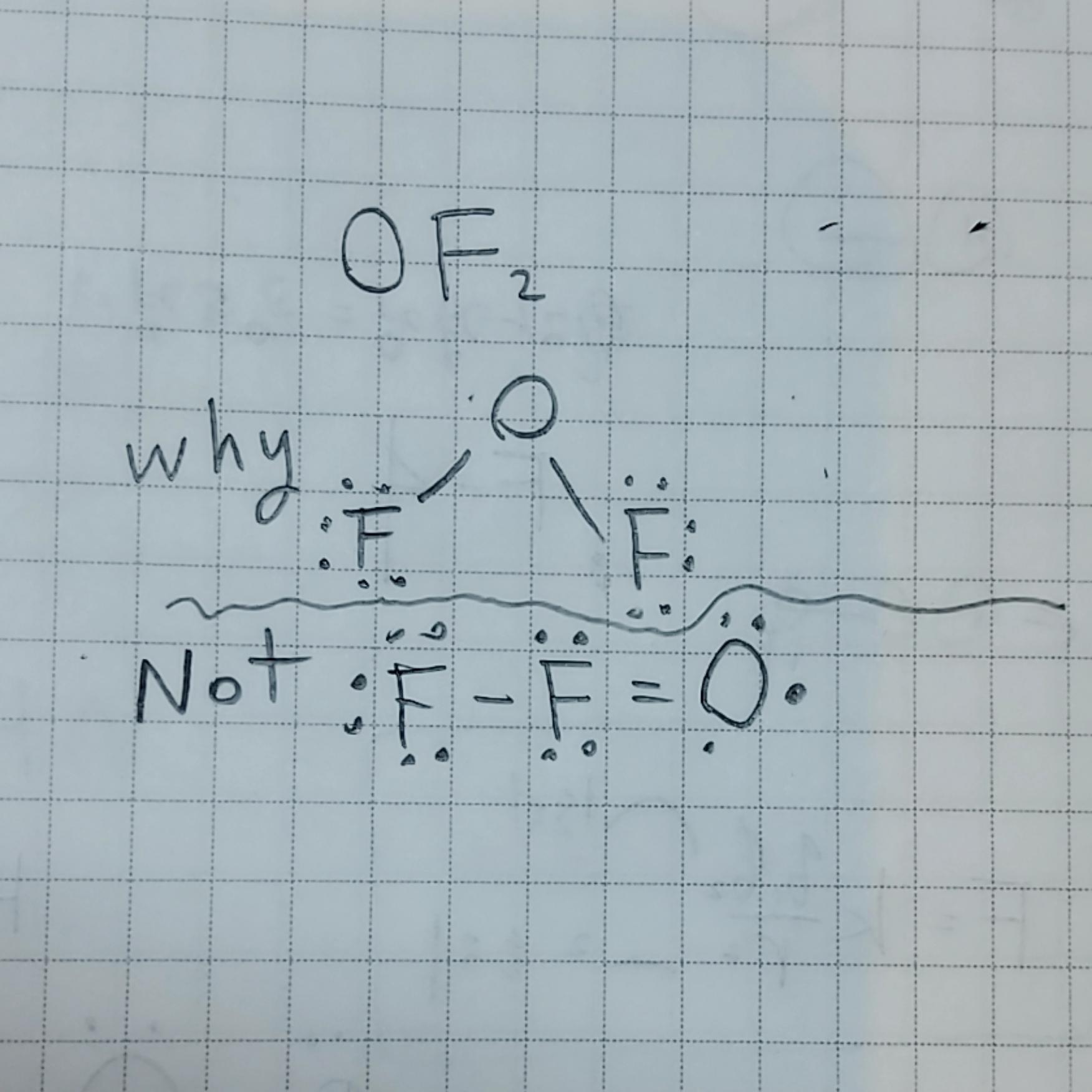
Oxygen difluoride has a bent molecular geometry, with the oxygen atom at the central position and the fluorine atoms positioned on either side. This arrangement gives OF2 its characteristic V-shaped structure. The molecule's polar nature arises from the difference in electronegativity between oxygen and fluorine, resulting in a partial negative charge on the oxygen atom and partial positive charges on the fluorine atoms.
The Significance of Oxygen Difluoride in Chemistry
Oxygen difluoride plays a vital role in various chemical processes and has garnered significant attention in academic and industrial research. Here are some key aspects highlighting its importance:
- Chemical Reactivity: OF2 is highly reactive due to the high electronegativity of fluorine atoms. It readily participates in a wide range of chemical reactions, making it a valuable reagent in synthesis and organic chemistry.
- Oxidizing Agent: Oxygen difluoride is a powerful oxidizing agent, capable of oxidizing many elements and compounds. This property finds applications in the production of fluorine-containing compounds and the synthesis of various organic compounds.
- Environmental Impact: While OF2 itself is not a major environmental concern, its reactivity and potential to form other fluorinated compounds raise environmental and health-related questions. Understanding its behavior is crucial for assessing its impact on ecosystems.
- Academic Research: The unique properties of oxygen difluoride make it an excellent subject for academic research. Scientists study its molecular structure, reactivity, and potential applications, contributing to our understanding of chemical principles and molecular behavior.
Step-by-Step Guide: Drawing Oxygen Difluoride
Now, let's dive into the practical part of this article and learn how to draw oxygen difluoride step by step. By following these simple instructions, you'll be able to visualize and represent this fascinating molecule effectively.
Step 1: Gather Your Materials
To draw oxygen difluoride, you'll need the following:
- A clean sheet of paper or a digital drawing platform (e.g., a graphics tablet and drawing software)
- A pencil or digital drawing tool
- An eraser for any necessary corrections
- A ruler or straightedge (optional, but recommended for precision)
Step 2: Construct the Basic Structure
Start by drawing a simple V-shaped structure to represent the molecular geometry of OF2. Begin with a vertical line, and then extend two arms at an angle from the top of the vertical line to form the V shape. Ensure that the arms are of equal length to maintain symmetry.
Label the central atom as "O" for oxygen and the two peripheral atoms as "F" for fluorine. This basic structure will serve as the foundation for your oxygen difluoride molecule.
Step 3: Add the Bonding and Electron Distribution
Now, it's time to add the chemical bonds and electron distribution to your drawing. Draw two single bonds connecting the oxygen atom to the fluorine atoms. Single bonds are represented by straight lines. Ensure that the bonds are evenly spaced and symmetrical.
To indicate the partial charges, you can add small symbols near the atoms. Place a "+" symbol near each fluorine atom to represent the partial positive charge, and a "-" symbol near the oxygen atom to indicate the partial negative charge. This step helps emphasize the polar nature of the molecule.
Step 4: Finalize and Enhance Your Drawing
At this stage, your oxygen difluoride molecule is almost complete. Take a step back and assess your drawing. Ensure that the bonds are correctly placed, the angles are accurate, and the overall structure is balanced. Use your eraser to make any necessary adjustments.
To enhance your drawing, you can add some shading or coloring to emphasize the three-dimensional nature of the molecule. Lightly shade the areas around the bonds to create a sense of depth and dimension. This step adds visual appeal and makes your drawing more engaging.
Congratulations! You've successfully drawn oxygen difluoride. Your illustration not only captures the molecule's unique structure but also highlights its polar nature. By following these simple steps, you can effectively represent this fascinating compound and explore its chemical properties further.
Conclusion: Unlocking the Beauty of Molecular Structures

Drawing chemical compounds like oxygen difluoride is not only an educational exercise but also a creative endeavor. It allows us to appreciate the intricate beauty of molecular structures and understand the fundamental principles of chemistry. By visualizing these compounds, we gain a deeper insight into their behavior and significance in various scientific fields.
As you continue your chemical journey, remember that each molecule tells a unique story. Exploring the world of chemistry through drawing and visualization opens up new perspectives and enhances our understanding of the microscopic world. So, keep exploring, keep learning, and continue to unlock the secrets of the chemical universe!
How stable is oxygen difluoride, and why is it unstable compared to other compounds?
+Oxygen difluoride is highly unstable due to the high electronegativity of fluorine atoms. This instability arises from the strong attraction between the fluorine atoms and the oxygen atom, leading to a highly reactive and unstable molecule. The bond between oxygen and fluorine is prone to breaking, making OF2 prone to decomposition and reactions with other substances.
What are some practical applications of oxygen difluoride in industry or research?
+Oxygen difluoride finds applications in the synthesis of various fluorinated compounds, including pharmaceuticals and agrochemicals. It is also used as an oxidizing agent in specific chemical processes. Additionally, its reactivity and unique properties make it a subject of interest in academic research, contributing to our understanding of chemical principles.
Are there any safety concerns associated with handling oxygen difluoride?
+Yes, oxygen difluoride is highly reactive and corrosive. It can cause severe burns and damage to skin and eyes upon contact. Inhalation can lead to respiratory irritation and potential long-term health effects. Therefore, handling oxygen difluoride requires proper safety equipment, including gloves, goggles, and a well-ventilated environment, to ensure the safety of those working with this compound.



