The Ultimate Guide to Hydroxide Ions

Welcome to the ultimate guide to hydroxide ions, a fascinating and crucial topic in chemistry. Hydroxide ions, often referred to as OH⁻, play a significant role in various chemical processes and have wide-ranging applications. In this comprehensive article, we will delve into the world of hydroxide ions, exploring their fundamental properties, chemical behavior, real-world applications, and their impact on our daily lives. So, let's embark on this journey to uncover the secrets of hydroxide ions and discover why they are so essential in the field of chemistry.
Understanding Hydroxide Ions: A Chemical Perspective
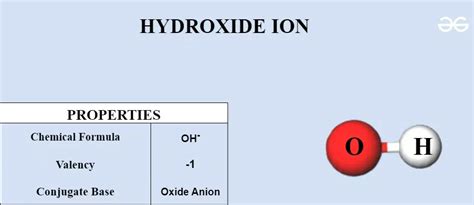
Hydroxide ions are negatively charged polyatomic ions composed of one oxygen atom and one hydrogen atom. The chemical formula for hydroxide ions is OH⁻, indicating the presence of one hydroxide ion with a negative charge. These ions are formed when water molecules dissociate into hydrogen ions (H⁺) and hydroxide ions (OH⁻) in aqueous solutions. This dissociation process is a fundamental concept in chemistry and forms the basis for many chemical reactions and reactions mechanisms.
The formation of hydroxide ions is governed by the autoionization of water, which is a self-ionization process where water molecules split into H⁺ and OH⁻ ions. This equilibrium reaction is represented as:
2H₂O ⇌ H₃O⁺ + OH⁻
In this equation, H₃O⁺ represents the hydronium ion, which is the hydrated form of the hydrogen ion. The autoionization of water is a dynamic process, and the equilibrium constant (Kw) for this reaction is temperature-dependent. At 25°C, the value of Kw is approximately 1.0 x 10⁻¹⁴, indicating that the concentration of hydroxide ions is relatively low compared to water molecules.
The presence of hydroxide ions in aqueous solutions has a profound impact on the pH of the solution. pH is a measure of the acidity or alkalinity of a solution, and it is determined by the concentration of hydrogen ions (H⁺) and hydroxide ions (OH⁻). Solutions with a higher concentration of hydroxide ions are considered basic or alkaline, while those with a higher concentration of hydrogen ions are acidic.
The pH scale, which ranges from 0 to 14, provides a quantitative measure of the acidity or alkalinity of a solution. Solutions with a pH of 7 are considered neutral, while those with a pH less than 7 are acidic, and those with a pH greater than 7 are basic. The pH scale is logarithmic, meaning that each unit change on the scale represents a tenfold difference in the concentration of hydrogen ions or hydroxide ions.
The Role of Hydroxide Ions in Chemical Reactions
Hydroxide ions are highly reactive and participate in a wide range of chemical reactions. They act as strong nucleophiles, which means they have a high affinity for electron-deficient centers, such as carbonyl groups (C=O) and electrophilic centers in other molecules. This nucleophilic behavior makes hydroxide ions crucial in various organic and inorganic reactions.
One of the most common reactions involving hydroxide ions is the hydrolysis reaction. Hydrolysis is the process of breaking down a compound using water. In the presence of hydroxide ions, certain chemical bonds can be cleaved, leading to the formation of new compounds. For example, the hydrolysis of esters in the presence of hydroxide ions results in the formation of carboxylic acids and alcohols.
Another important reaction involving hydroxide ions is the formation of salts. When a strong acid reacts with a strong base, such as hydroxide ions, the resulting product is a salt and water. This neutralization reaction is often used to control the pH of solutions and has practical applications in various industries, such as water treatment and chemical manufacturing.
Applications of Hydroxide Ions in Everyday Life
Hydroxide ions have numerous applications in our daily lives, ranging from household cleaning to industrial processes. One of the most well-known uses of hydroxide ions is in household cleaners, particularly in the form of sodium hydroxide (NaOH), commonly known as caustic soda or lye.
Sodium hydroxide is a strong base and a powerful cleaning agent. It is used in various cleaning products, such as drain cleaners, oven cleaners, and laundry detergents. The hydroxide ions in sodium hydroxide react with grease, oils, and other organic compounds, breaking them down into simpler substances that can be easily rinsed away. This cleaning action makes sodium hydroxide an essential component in maintaining hygiene and cleanliness in our homes.
In addition to household cleaning, hydroxide ions are also utilized in industrial processes. One notable application is in the production of paper. The papermaking process involves the use of hydroxide ions to convert wood pulp into a usable form. Hydroxide ions help break down the complex cellulose fibers, making them more amenable to processing and forming into paper.
Another industrial application of hydroxide ions is in the production of biodiesel. Biodiesel is a renewable and environmentally friendly fuel derived from vegetable oils or animal fats. The process of converting these oils into biodiesel involves the use of hydroxide ions as a catalyst. Hydroxide ions facilitate the transesterification reaction, which converts the oils into fatty acid methyl esters (biodiesel) and glycerol.
Environmental Impact and Safety Considerations
While hydroxide ions have numerous beneficial applications, it is essential to consider their environmental impact and safety aspects. Strong bases, such as sodium hydroxide, can be highly corrosive and pose a risk to human health and the environment if not handled properly.
In industrial settings, the proper handling and disposal of hydroxide ions and their derivatives are crucial to minimize environmental pollution. Strict regulations and guidelines are in place to ensure the safe handling and disposal of these substances. Additionally, researchers and scientists are continuously working on developing more sustainable and environmentally friendly alternatives to traditional hydroxide-based processes.
When it comes to personal safety, it is important to handle hydroxide-based cleaning products with caution. These products should be kept out of reach of children and pets, and proper protective equipment, such as gloves and eye protection, should be worn during their use. Additionally, adequate ventilation is necessary to minimize exposure to any fumes or vapors.
Hydroxide Ions in Nature and Biological Systems

Hydroxide ions are not only significant in chemical reactions and industrial processes but also play a vital role in natural and biological systems. In fact, the presence and concentration of hydroxide ions in various environments can have a profound impact on the functioning of ecosystems and biological organisms.
The Role of Hydroxide Ions in Aquatic Environments
Aquatic environments, such as lakes, rivers, and oceans, are home to a diverse array of plant and animal life. The pH of these water bodies is a critical factor that influences the health and survival of these organisms. Hydroxide ions, along with hydrogen ions, contribute to the pH regulation of aquatic environments.
In natural waters, the pH is typically slightly basic, ranging from 6.5 to 8.5. This slightly basic pH is essential for the growth and development of aquatic plants and the survival of fish and other aquatic organisms. Hydroxide ions help maintain this optimal pH range by neutralizing excess hydrogen ions and regulating the acidity of the water.
However, human activities can disrupt the natural pH balance of aquatic environments. For example, industrial waste and agricultural runoff often contain high levels of pollutants, including acids or bases, which can alter the pH of water bodies. This alteration in pH can have detrimental effects on aquatic life, leading to reduced biodiversity, decreased growth rates, and even mass die-offs of sensitive species.
To mitigate the impact of human activities on aquatic environments, water quality monitoring and pH regulation measures are implemented. These measures involve the careful control and management of wastewater discharges, as well as the implementation of pH adjustment strategies. Hydroxide ions, in the form of alkaline substances like calcium hydroxide (Ca(OH)₂), are often used to neutralize acidic wastewaters and restore the pH balance of aquatic ecosystems.
Hydroxide Ions and Biological Processes
Hydroxide ions are not only important in aquatic environments but also play a crucial role in various biological processes. In the human body, for instance, hydroxide ions are involved in maintaining the pH balance of body fluids, including blood and intracellular fluids.
The human body tightly regulates its pH to ensure optimal functioning of enzymes, proteins, and other biological molecules. The presence of hydroxide ions helps buffer the pH of body fluids, preventing extreme fluctuations that could be detrimental to cellular processes. For example, the bicarbonate buffer system, which involves the conversion of carbon dioxide (CO₂) into bicarbonate ions (HCO₃⁻) and hydroxide ions (OH⁻), helps maintain the pH of blood within a narrow range of 7.35 to 7.45.
Additionally, hydroxide ions are involved in digestive processes. In the stomach, gastric juices contain hydrochloric acid (HCl), which creates an acidic environment to aid in the breakdown of food. However, the stomach lining itself must be protected from this acidic environment. To achieve this, the stomach secretes a layer of mucus, which contains bicarbonate ions (HCO₃⁻) that react with hydrogen ions (H⁺) to form water and hydroxide ions (OH⁻), neutralizing the acid and preventing damage to the stomach lining.
In summary, hydroxide ions are vital in maintaining the pH balance of aquatic environments and biological systems. Their presence and concentration play a critical role in the health and functioning of ecosystems and living organisms. By understanding the role of hydroxide ions in these natural and biological contexts, we can better appreciate the delicate balance of our environment and strive to protect it.
The Future of Hydroxide Ions: Innovations and Advances
As our understanding of hydroxide ions and their applications continues to evolve, so does the potential for innovative advancements in various fields. Researchers and scientists are exploring new ways to harness the power of hydroxide ions and develop sustainable and efficient processes that can benefit society and the environment.
Emerging Technologies and Sustainable Applications
One area of focus in the development of hydroxide-based technologies is sustainability. Researchers are working on creating more environmentally friendly alternatives to traditional hydroxide-based processes. For example, in the field of energy storage, hydroxide ions are being explored as a key component in advanced batteries, such as zinc-air batteries and alkaline fuel cells.
Zinc-air batteries, which use hydroxide ions as an electrolyte, offer high energy density and the potential for long-term energy storage. These batteries have the advantage of being reusable and environmentally friendly, as zinc is a readily available and recyclable material. Additionally, alkaline fuel cells, which utilize hydroxide ions as a catalyst, provide efficient and clean energy generation, making them a promising option for sustainable power generation.
In the field of water treatment, innovative hydroxide-based technologies are being developed to address the challenges of water scarcity and pollution. One such technology is the use of hydroxide ions in advanced oxidation processes (AOPs). AOPs involve the generation of highly reactive oxygen species, such as hydroxyl radicals (OH⁻), which can effectively degrade organic pollutants and microorganisms in water. This approach offers a promising solution for the treatment of contaminated water sources and the removal of harmful substances.
Advancements in Materials Science
Hydroxide ions also play a significant role in materials science, particularly in the development of advanced materials with unique properties. One notable example is the use of hydroxide ions in the synthesis of nanomaterials, such as metal oxide nanoparticles. These nanoparticles have a wide range of applications, including in electronics, catalysis, and medicine.
In electronics, metal oxide nanoparticles can be used as transparent conductive coatings, enhancing the performance of devices such as solar cells and displays. In catalysis, hydroxide-based nanoparticles can serve as highly efficient catalysts, facilitating chemical reactions with reduced energy requirements and improved selectivity. In medicine, these nanoparticles have shown potential in targeted drug delivery and tissue engineering applications, offering new possibilities for the treatment of various diseases.
Furthermore, hydroxide ions are being explored in the development of advanced energy storage materials. For instance, research is underway to create hydroxide-based electrode materials for lithium-ion batteries. These materials offer improved electrochemical performance, higher energy density, and longer cycle life, which could revolutionize the field of energy storage and contribute to the widespread adoption of electric vehicles and renewable energy sources.
Hydroxide Ions and the Future of Chemistry
The future of hydroxide ions in chemistry is filled with exciting possibilities. As our understanding of hydroxide ions and their behavior deepens, new chemical reactions and mechanisms are being discovered. These advancements are opening up avenues for the development of novel materials, improved industrial processes, and more sustainable practices.
One area of focus is the exploration of hydroxide ions in green chemistry. Green chemistry aims to minimize the environmental impact of chemical processes by designing sustainable and eco-friendly alternatives. Hydroxide ions, with their nucleophilic and basic properties, can be utilized in various green chemical reactions, such as biocatalysis and enzymatic processes. By harnessing the power of hydroxide ions in a controlled manner, researchers can develop more efficient and environmentally conscious chemical processes.
Additionally, the study of hydroxide ions is contributing to the advancement of analytical chemistry and the development of new analytical techniques. Hydroxide ions can serve as sensitive indicators of pH and are used in various pH measurement devices, such as pH meters and pH probes. These devices play a crucial role in quality control, environmental monitoring, and biomedical research, enabling precise and accurate pH measurements.
In summary, the future of hydroxide ions holds immense potential for innovation and advancements across various fields. From sustainable energy storage to advanced materials and green chemistry, hydroxide ions are poised to play a pivotal role in shaping the future of science and technology. By harnessing their unique properties and exploring their applications, we can continue to push the boundaries of what is possible and create a more sustainable and prosperous world.
What are the common sources of hydroxide ions in nature?
+Hydroxide ions can be found in various natural sources, including rainwater, which contains dissolved hydroxide ions from atmospheric gases. Additionally, hydroxide ions are present in volcanic gases, hot springs, and geothermal waters. These natural sources contribute to the hydroxide ion concentration in different environments.
How do hydroxide ions affect the pH of solutions?
+Hydroxide ions have a direct impact on the pH of solutions. The concentration of hydroxide ions is inversely related to the pH. As the concentration of hydroxide ions increases, the pH becomes more basic, resulting in a higher pH value. Conversely, a decrease in hydroxide ion concentration leads to a more acidic solution with a lower pH.
What are some common safety precautions when working with hydroxide-based substances?
+When handling hydroxide-based substances, it is important to take appropriate safety precautions. These may include wearing protective clothing, such as gloves and eye protection, to prevent skin and eye contact. Adequate ventilation is necessary to minimize exposure to fumes or vapors. Additionally, proper storage and disposal practices should be followed to avoid accidents and environmental contamination.
Can hydroxide ions be used in green chemistry applications?
+Yes, hydroxide ions have significant potential in green chemistry applications. Their nucleophilic and basic properties can be harnessed in various sustainable chemical reactions, such as biocatalysis and enzymatic processes. By utilizing hydroxide ions in a controlled manner, researchers can develop more eco-friendly and efficient chemical processes.



