The Lewis Structure Revealed: CCL2F2
The Intriguing Molecular World: Exploring the Lewis Structure of CCL2F2
In the vast realm of molecular structures, we find intriguing compounds like CCL2F2, a unique molecule that has piqued the interest of many chemists and scientists. This article aims to delve into the details of CCL2F2’s Lewis structure, unraveling its mysteries and shedding light on its chemical nature.
CCL2F2, also known as Chloropentafluoroethane, is a fluorocarbon commonly used as a refrigerant in various industrial applications. Its molecular formula, CCl2F2, hints at its complex structure, which we will explore in depth. This molecule's significance lies not only in its practical applications but also in the insights it offers into the fundamental principles of chemical bonding and molecular geometry.
As we embark on this journey through the molecular landscape, we will uncover the precise arrangement of atoms, the nature of chemical bonds, and the unique properties that arise from this specific molecular structure. By understanding the Lewis structure of CCL2F2, we can gain a deeper appreciation for the intricate dance of atoms that forms the basis of our physical world.
The Building Blocks: Atoms and Their Arrangement
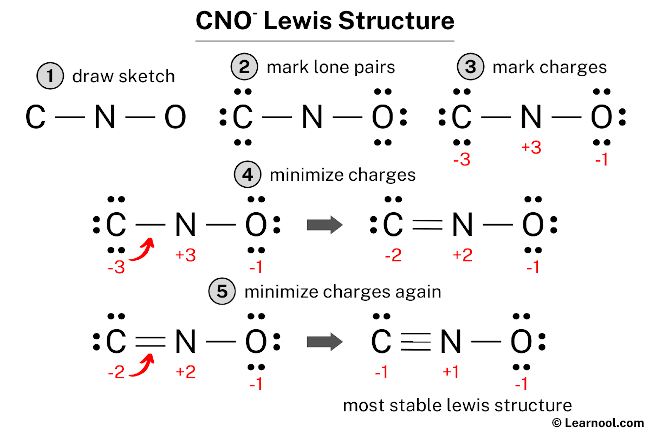
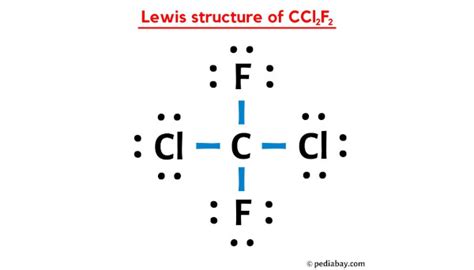
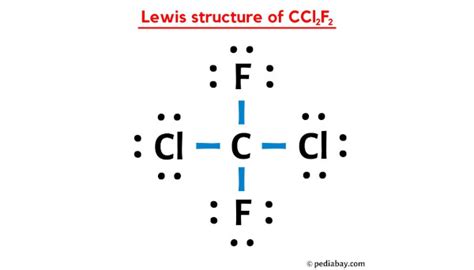
The Lewis structure of CCL2F2 is a visual representation of how the atoms in this molecule are arranged and how they bond with each other. At its core, CCL2F2 is composed of one carbon atom, two chlorine atoms, and two fluorine atoms. The carbon atom acts as the central hub, with the other atoms forming a unique arrangement around it.
The carbon atom, with its four valence electrons, is crucial to the stability of the molecule. It forms four single bonds, two with the chlorine atoms and two with the fluorine atoms. This arrangement satisfies the octet rule, ensuring that each atom has a complete valence shell.
The chlorine atoms, with their seven valence electrons, contribute to the overall stability of CCL2F2. They form single bonds with the carbon atom, completing their octets. The fluorine atoms, on the other hand, with their one valence electron, are highly electronegative. They form strong single bonds with the carbon atom, creating a highly stable molecular structure.
The Role of Electronegativity
The electronegativity of the fluorine atoms is a key factor in the unique properties of CCL2F2. With their high electronegativity, fluorine atoms attract electrons towards themselves, creating a highly polarized bond with the carbon atom. This polarization results in a significant dipole moment, which influences the molecule's overall behavior.
The difference in electronegativity between the carbon and fluorine atoms creates a partial negative charge on the fluorine atoms and a partial positive charge on the carbon atom. This charge separation is crucial for the molecule's reactivity and its ability to interact with other substances.
| Atom | Electronegativity |
|---|---|
| Carbon (C) | 2.55 |
| Chlorine (Cl) | 3.16 |
| Fluorine (F) | 3.98 |
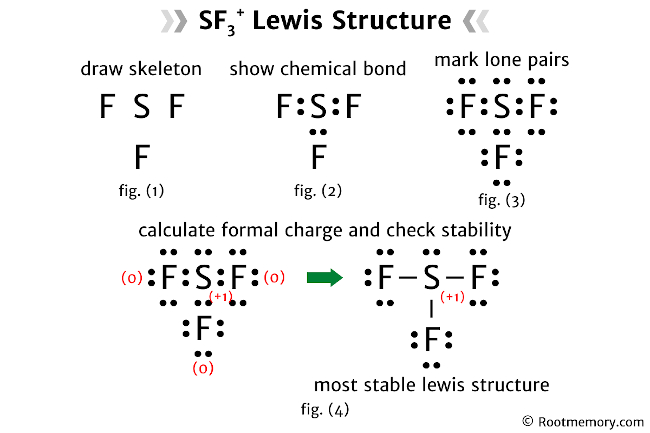
Bonding and Molecular Geometry
The Lewis structure of CCL2F2 reveals a tetrahedral molecular geometry. This geometry arises from the arrangement of the four single bonds around the central carbon atom. The chlorine and fluorine atoms are positioned at the corners of a tetrahedron, with the carbon atom at the center.
The tetrahedral geometry is a result of the carbon atom's need to maximize its bond angles and minimize electronic repulsion. This arrangement ensures that the molecule is stable and has minimal strain on its bonds.
The bond angles in CCL2F2 are approximately 109.5 degrees, a common angle found in tetrahedral molecules. These angles optimize the electronic interactions within the molecule, contributing to its overall stability.
Bond Types and Strength
CCL2F2 exhibits strong single bonds between its atoms. The carbon-chlorine and carbon-fluorine bonds are both sigma (σ) bonds, formed by the head-on overlap of atomic orbitals. These bonds are highly stable and contribute to the molecule's overall strength and rigidity.
The bond length between the carbon and chlorine atoms is approximately 1.76 Å (Angstroms), while the carbon-fluorine bond length is slightly shorter at around 1.33 Å. These bond lengths are characteristic of single bonds between these specific atoms.
| Bond | Bond Type | Bond Length (Å) |
|---|---|---|
| Carbon-Chlorine | Sigma (σ) | 1.76 |
| Carbon-Fluorine | Sigma (σ) | 1.33 |
The Impact of CCL2F2's Structure
The unique Lewis structure of CCL2F2 has significant implications for its physical and chemical properties. The molecule's high stability, due to its strong bonds and optimal geometry, makes it an excellent choice for various industrial applications, particularly as a refrigerant.
The polarized nature of the carbon-fluorine bonds also contributes to CCL2F2's reactivity. This molecule can undergo various chemical reactions, including nucleophilic substitution and addition reactions, making it a versatile compound in the chemical industry.
Furthermore, the molecule's high electronegativity and polar nature make it an effective solvent for certain reactions. CCL2F2 can dissolve and react with a range of substances, facilitating chemical processes and offering new possibilities for synthesis and extraction.
Environmental Considerations
While CCL2F2 has proven to be a valuable compound in various industries, its environmental impact cannot be overlooked. As a fluorocarbon, CCL2F2 has a high global warming potential (GWP) and can contribute to ozone depletion if not handled and disposed of properly.
However, with responsible use and proper regulations, the benefits of CCL2F2 can be maximized while minimizing its environmental footprint. The chemical industry is continuously working on developing sustainable practices and alternatives to ensure the safe and responsible use of such compounds.
Frequently Asked Questions
What is the main use of CCL2F2?
+
CCL2F2, also known as Chloropentafluoroethane, is primarily used as a refrigerant in various industrial applications. Its stability, high boiling point, and non-flammability make it an ideal choice for cooling systems.
How does the Lewis structure of CCL2F2 affect its reactivity?
+
The Lewis structure of CCL2F2, with its polarized carbon-fluorine bonds, influences its reactivity. The high electronegativity of fluorine atoms creates a partial negative charge, making the molecule more susceptible to nucleophilic attacks and substitution reactions.
Are there any environmental concerns associated with CCL2F2’s use?
+
Yes, as a fluorocarbon, CCL2F2 has a high global warming potential and can contribute to ozone depletion if not handled and disposed of properly. Responsible use and adherence to environmental regulations are crucial to mitigate its environmental impact.
What are the key advantages of CCL2F2 over other refrigerants?
+
CCL2F2 offers several advantages over traditional refrigerants. It has a higher boiling point, making it suitable for a wider range of temperatures. It is also non-flammable and has a low toxicity level, making it safer to handle and use.