Water's Boiling Point: 3 Key Facts
Water, a seemingly simple substance, plays a vital role in our daily lives and is a fundamental component of life on Earth. Among its many unique properties, the boiling point of water stands out as a crucial characteristic, impacting various scientific, industrial, and everyday processes. In this article, we will delve into the fascinating world of water's boiling point, uncovering three key facts that reveal the significance of this phenomenon.
The Intricate Nature of Water’s Boiling Point

Water’s boiling point is a multifaceted concept that extends beyond the commonly known temperature of 100°C or 212°F. It is a threshold where water transitions from its liquid state to vapor, and this process is influenced by a myriad of factors, each contributing to the complexity of this natural phenomenon.
Fact 1: Atmospheric Pressure is a Key Determinant
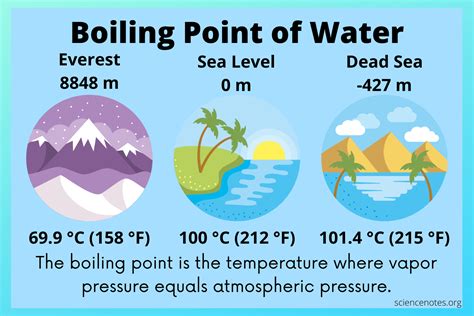
One of the most critical factors influencing water’s boiling point is atmospheric pressure. At sea level, water boils at approximately 100°C. However, as you ascend to higher altitudes, the atmospheric pressure decreases, and consequently, water requires less energy to reach its boiling point. This means that at higher elevations, water boils at a lower temperature. For instance, at an altitude of 1,500 meters (4,921 feet), water boils at around 95°C (203°F). This phenomenon is not limited to mountainous regions; even small changes in altitude can impact water’s boiling point. Therefore, when cooking or performing experiments, understanding the effect of altitude on water’s boiling point is essential for accurate results.
Consider the experience of a chef preparing a gourmet meal in a high-altitude city like Denver, Colorado, which sits at an elevation of 1,609 meters (5,280 feet). The lower boiling point of water at this altitude will affect the cooking time and temperature of various dishes, requiring adjustments to recipes to ensure the desired outcomes. This illustrates how water's boiling point, influenced by atmospheric pressure, can have real-world implications in the culinary world.
Fact 2: The Role of Impurities in Boiling Point Elevation
In pure water, the boiling point is a constant and well-defined temperature. However, the presence of impurities, such as salts, minerals, or other dissolved substances, can lead to a phenomenon known as boiling point elevation. This occurs because these impurities disrupt the normal behavior of water molecules, requiring a higher temperature for the water to reach its boiling point. The extent of boiling point elevation depends on the type and concentration of the impurities present.
For example, seawater, with its high salt content, has a significantly elevated boiling point compared to pure water. This property is utilized in desalination processes, where seawater is boiled to separate pure water from the salts and other impurities. By understanding the impact of impurities on water's boiling point, scientists and engineers can design more efficient and effective processes for various industries, including water treatment and chemical manufacturing.
Fact 3: Superheating: A Fascinating Phenomenon
Superheating is a unique and somewhat mysterious behavior exhibited by water and other liquids. It occurs when water is heated beyond its boiling point without actually boiling. This can happen in situations where water is heated rapidly or in a smooth, clean container, preventing the formation of bubbles necessary for boiling. Superheated water can exist in a metastable state, appearing calm on the surface but ready to violently boil when disturbed.
A classic demonstration of superheating involves heating water in a microwave oven. If the water is heated without any impurities or disturbances, it can reach temperatures significantly higher than its normal boiling point. However, introducing a foreign object, such as a spoon or sugar cube, can trigger a sudden and vigorous boiling reaction, known as a "superheat explosion." While this phenomenon can be fascinating to observe, it also highlights the importance of understanding the potential risks associated with superheating, particularly in industrial processes where rapid and uncontrolled boiling could lead to dangerous outcomes.
| Factor | Effect on Boiling Point |
|---|---|
| Atmospheric Pressure | Lower pressure results in a lower boiling point. |
| Impurities | Elevates the boiling point based on the type and concentration of impurities. |
| Superheating | Heating water beyond its boiling point without actual boiling. |
How does altitude affect the boiling point of water, and why is this important for cooking and scientific experiments?
+
Altitude affects the boiling point of water due to changes in atmospheric pressure. At higher altitudes, the lower pressure allows water to boil at a lower temperature. This is important for cooking because it can impact cooking times and temperatures, requiring adjustments to recipes. In scientific experiments, understanding this relationship is crucial for accurate results, especially in fields like chemistry and physics.
What is boiling point elevation, and how does it impact industrial processes like desalination?
+
Boiling point elevation is the phenomenon where the presence of impurities in water raises its boiling point. This is significant for desalination processes because seawater, with its high salt content, has an elevated boiling point. By understanding and utilizing this property, engineers can design more efficient desalination systems to produce fresh water from seawater.
What is superheating, and what are some potential risks associated with it?
+
Superheating is the state where water is heated beyond its boiling point without actually boiling. It can occur in smooth, undisturbed containers. The risk lies in the sudden and violent boiling that can occur when superheated water is disturbed, potentially leading to scalding or damage in industrial settings. Awareness and proper handling procedures are essential to mitigate these risks.



